A simple COVID-19 test kit combines virus amplification with a CRISPR-Cas system for effective SARS-CoV-2 detection. The kit, called iSCAN, uses reagents that can be locally manufactured.
“Our whole iSCAN procedure can be completed in less than an hour and can be easily adopted as a point-of-care detection system at airports and borders,” says KAUST Ph.D. student Ahmed Mahas.
The current gold standard in SARS-CoV-2 testing is the PCR test, in which DNA primers recognize specific RNA sequences in the viral genome that are then copied using a specific enzyme. This “amplification” process makes it easier to detect the originally small amounts of viral RNA present in the nasopharyngeal swabs taken from patients. This test can reliably detect if a person really has the virus without providing too many false positive or negative results. But it needs highly skilled personnel to conduct the test, which is done in multiple steps in central laboratories with sophisticated equipment.
iSCAN, developed by a team led by KAUST bioengineer Magdy Mahfouz overcomes many of the disadvantages of the PCR test while providing relatively trustworthy results.
Significantly, the test’s reagents were manufactured at KAUST. This includes the enzymes needed for amplification and another enzyme that specifically detects viral sequences within the copied material. The availability of reagents and equipment has been a huge obstacle since the start of the COVID-19 pandemic.
To use iSCAN, the contents from a patient’s sample, collected with a nasopharyngeal swab, are placed in a small test tube containing the DNA primers and enzymes that can amplify SARS-CoV-2 genetic material. The contents are incubated at a temperature of 62 degrees Celsius for half an hour. This process is referred to as RT-LAMP. Once enough viral RNA is amplified, a droplet containing the enzyme Cas12 is added to the mix and left for another 15 minutes. This enzyme only recognizes viral RNA belonging to SARS-CoV-2, overcoming an issue with RT-LAMP, where false amplification and cross-contamination can be a problem.
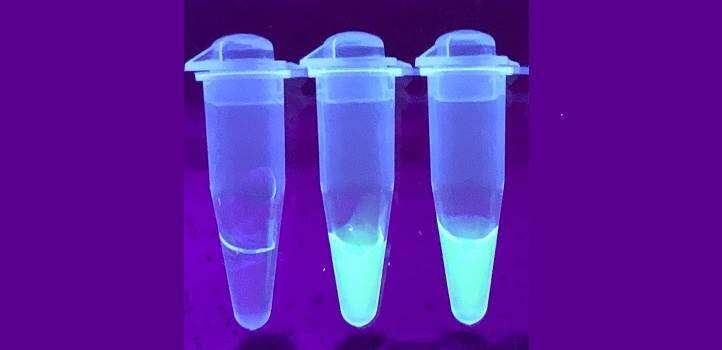
Finally, one of two methods can be used to visualize the result. One involves shining ultraviolet light on the sample, with a detector analyzing the light coming out from it to report the amount of viral RNA. The other approach involves inserting specially designed strips into the tubes, similar to those used in pregnancy tests. Both approaches work well, although the ultraviolet light method provided more accurate results.
Source: Read Full Article
