A new study suggests a new coronavirus test developed by scientists from the University of Washington and the Brotman Baty Institute for Precision Medicine produces faster and more sensitive results than conventional PCR tests to measure severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA.
The RT-LAMP test kit known as “Harmony COVID-19” is a point-of-care test designed to be inexpensive and straightforward to use with the ability to examine up to four samples at once. The device is intended to better help healthcare workers in identifying SARS-CoV-2 in patients.
The researchers also hope to extend Harmony COVID-19 for rapid testing of other respiratory infections, including seasonal SARS-CoV-2, influenza, and RSV.
The team writes:
“Tests such as Harmony will become a common practice for confirming the presence of viral infections that can be treated with antiviral therapies or that demand infection control, as well as helping to reduce inappropriate use of antibiotics. Further, test platforms developed for this pandemic could be more readily adapted to detect new diseases to increase readiness for future pandemics.”
The study “Harmony COVID-19: a ready-to-use kit, low-cost detector, and smartphone app for point-of-care SARS-CoV-2 RNA detection” is published on the preprint medRxiv* server.
How they did it
The Harmony COVID-19 test kit was assessed in the current study by having healthcare workers measure the RNA of contrived specimens from the nasal and saliva matrix. In addition, healthcare workers also measured RNA from stored clinical samples.
Fresh clinical samples could not be obtained in time for the study, which the authors acknowledge is a limitation of the study.
Healthcare workers learned how to use the test through some written instructions and a video detailing the procedure.
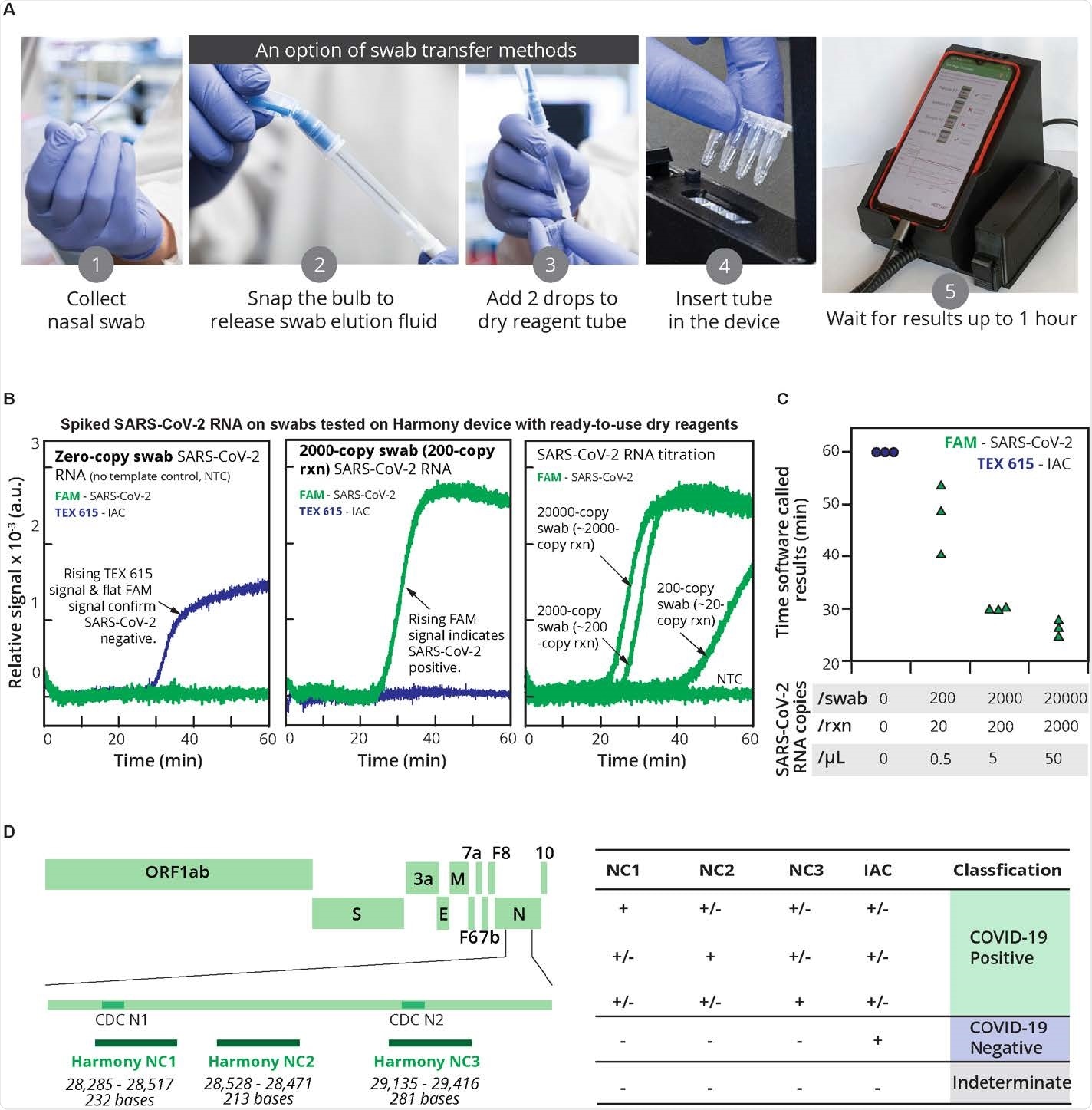
Results suggest high accuracy and sensitivity in testing assay
Testing of Harmony COVID-19 produced an inexpensive and straightforward rapid test with features similar to conventional laboratory-based tests. Healthcare workers had a 95% accuracy while using the assay.
Results also showed the Harmony COVID-19 testing kit had high sensitivity.
Ready-to-use reagents in the testing kit helped with specificity and accuracy. The assay detected about 15 copies/reaction of synthetic SARS-CoV-2 RNA and real RNA samples collected at more than 20 copies/reaction with no false positives. The test was also able to give results for high viral load samples in 17 minutes.
The test also successfully detected samples with more than 20 viral particles in the human nasal matrix. In addition, the test had moderate sensitivity for saliva at similar concentrations.
Specifically, about 97% of contrived samples in the nasal matrix was detected, and 83% of samples were detected in saliva.
“Our current level of assay sensitivity would be sufficient to detect SARS-CoV-2 in most nasal specimens (~103 to ~109 copies/swab) or saliva (~104 to ~108 copies/mL) infected individuals during the first week after the onset,” explained the research team.
The researchers attributed the test’s high sensitivity and specificity to using a novel polymerase and probe design to specify between SARS-CoV-2 from IAC amplification.
Improvements to the assay
The researchers point out that the testing assay could be modified to increase the detection of SARS-CoV-2. They explain that conducting the test at a cooler and more optimal temperature for the reverse transcriptase enzyme followed by a higher temperature for DNA amplification could potentially increase the speed or sensitivity of SARS-CoV-2 detection.
Using an RNA internal amplification control that more accurately reflects the SARS-CoV-2 virus and that can be packaged in a viral envelope could also improve results. The current study had the Harmony COVID-19 assay use a DNA internal amplification control, which fails to grasp lysis and cDNA conversion processes.
“The advances in awareness and acceptance of testing for infections at scale, and the technology platforms developed will have an ongoing benefit for COVID-19 control, reducing harm from other endemic diseases, and fighting future pandemics,” concluded the research team.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Panpradist N, et al. Harmony COVID-19: a ready-to-use kit, low-cost detector, and smartphone app for point-of-care SARS-CoV-2 RNA detection. medRxiv, 2021. doi: https://doi.org/10.1101/2021.08.12.21261875, https://www.medrxiv.org/content/10.1101/2021.08.12.21261875v1
Posted in: Device / Technology News | Medical Research News | Disease/Infection News
Tags: Assay, Cell, Coronavirus, Coronavirus Disease COVID-19, Diagnostic, DNA, Enzyme, Gene, Healthcare, Infection Control, Influenza, Laboratory, Medicine, Pandemic, Polymerase, Reagents, Research, Respiratory, Reverse Transcriptase, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Virus

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article
