According to ARRS’ American Journal of Roentgenology (AJR), a reduced dose of gadobutrol is non-inferior to 100%-standard dose of gadoterate for contrast-enhanced brain MRI.
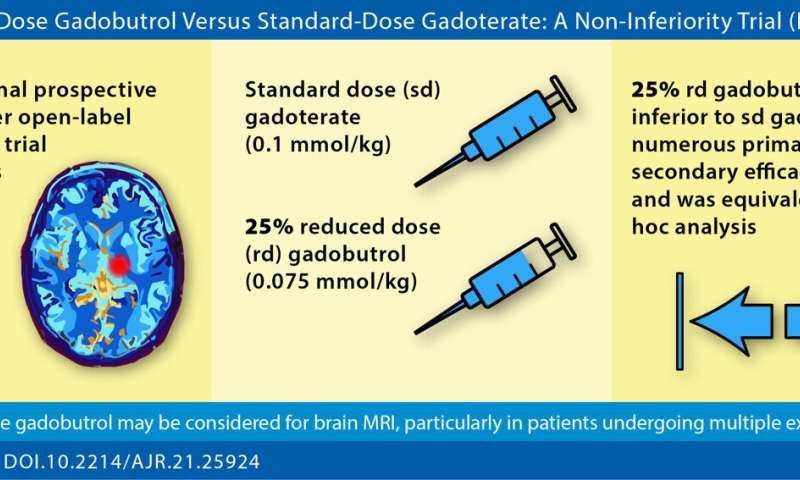
“A 25% reduced gadobutrol dose demonstrated non-inferior efficacy versus standard dose gadoterate for contrast-enhanced brain MRI,” corresponding author Jan Endrikat of Germany’s University Medical School of Saarland elaborated, “warranting particular consideration in patients undergoing multiple contrast-enhanced examinations.”
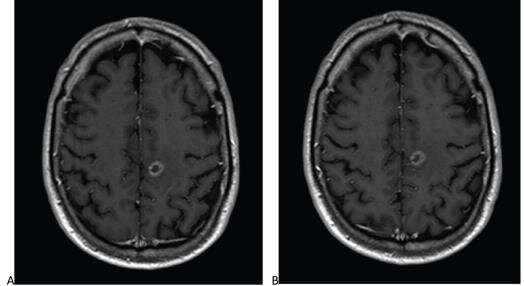
In this international, prospective, multicenter, open-label, crossover trial (LEADER-75), 141 patients (78 men, 63 women; mean age, 58.5 years) with known or suspected CNS pathology underwent contrast-enhanced brain MRI with standard-dose gadoterate (0.1 mmol/ kg). If an enhancing lesion was identified, a second MRI with reduced-dose gadobutrol (0.075 mmol/kg) was performed within 15 days.
Comparison of reduced-dose gadobutrol and standard-dose gadoterate versus unenhanced imaging demonstrated noninferiority using 20% margin for three primary efficacy measures: subjective lesion enhancement, lesion border delineation, lesion internal morphology.
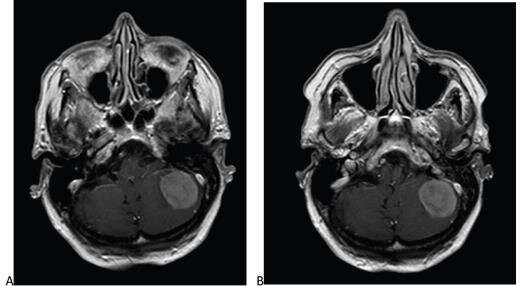
Furthermore, in post-hoc analysis, mean readings for subjective lesion enhancement, lesion border delineation, and lesion internal morphology differed by less than 1%—supporting equivalence using a narrow ±5% margin.
“Various secondary variables also supported non-inferiority of reduced-dose gadobutrol,” the authors of the AJR article added.
Source: Read Full Article
