Is one COVID vaccine better than the other? Four current vaccines — Moderna, Pfizer-BioNTech, Johnson & Johnson, and AstraZeneca — are highly effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but a new medRxiv* study suggests the level of protection varies.
.jpg)
Researchers from The Netherlands found the mRNA vaccines produce more antibodies than adenovirus-based vaccines.
“The implication is that individuals receiving one of the adenovirus vector-based vaccines are more vulnerable to infection with the Beta and Delta VOCs, which is consistent with the lower efficacy of these vaccines against symptomatic infection with VOCs compared to the mRNA vaccines, although all vaccines are highly effective at preventing severe disease by VOCs,” explained the researchers.
Against SARS-CoV-2 variants, especially the Beta variant, immunity weakened most among adenovirus vector-based vaccines.
Overview of experiment
The researchers collected convalescent plasma samples of individuals who were vaccinated with either the Pfizer-BioNTech (n = 50), Moderna (n = 40), AstraZeneca (n = 41), or the single-shot Johnson & Johnson vaccine (n = 16).
Participants included healthcare workers who were never exposed to SARS-CoV-2. The four vaccine groups were fairly similar in composition; 62-87% were female, with the majority between 35 and 60 years old. However, the majority of individuals who were vaccinated with the two-dose AstraZeneca shot were over 60 years of age. The age difference is due to the Dutch government allocating most AstraZeneca shots to this age group.
Neutralizing antibodies were measured from serum samples and tested against four SARS-CoV-2 variants of concern — Alpha, Beta, Gamma, and Delta.
Serum samples were collected at least 3 weeks after the first vaccination and 4 weeks after the second vaccination. For the one-dose Johnson & Johnson shot, samples were collected 5 to 8 after vaccination.
Both mRNA vaccines show superior humoral immune response
All four vaccines showed antibody responses against the spike protein of the original coronavirus strain.
Four weeks after full dosing, mRNA vaccines had significantly higher neutralization titers compared to adenovirus-based vaccines. However, antibody responses decreased in individuals who received the AstraZeneca and the Johnson & Johnson vaccine.
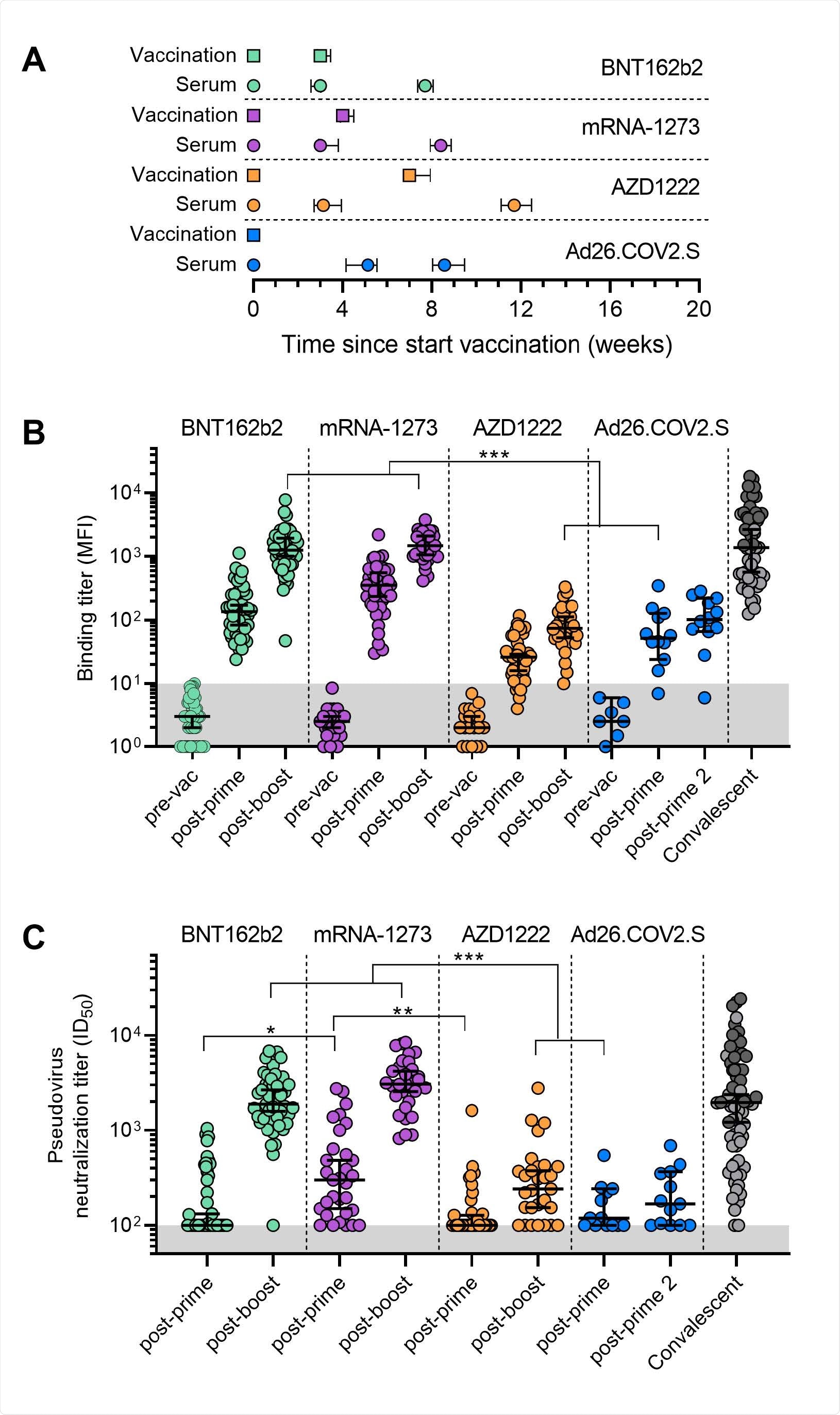
The AstraZeneca and the Johnson & Johnson vaccine had a 17 to 29-fold reduction in antibody responses.
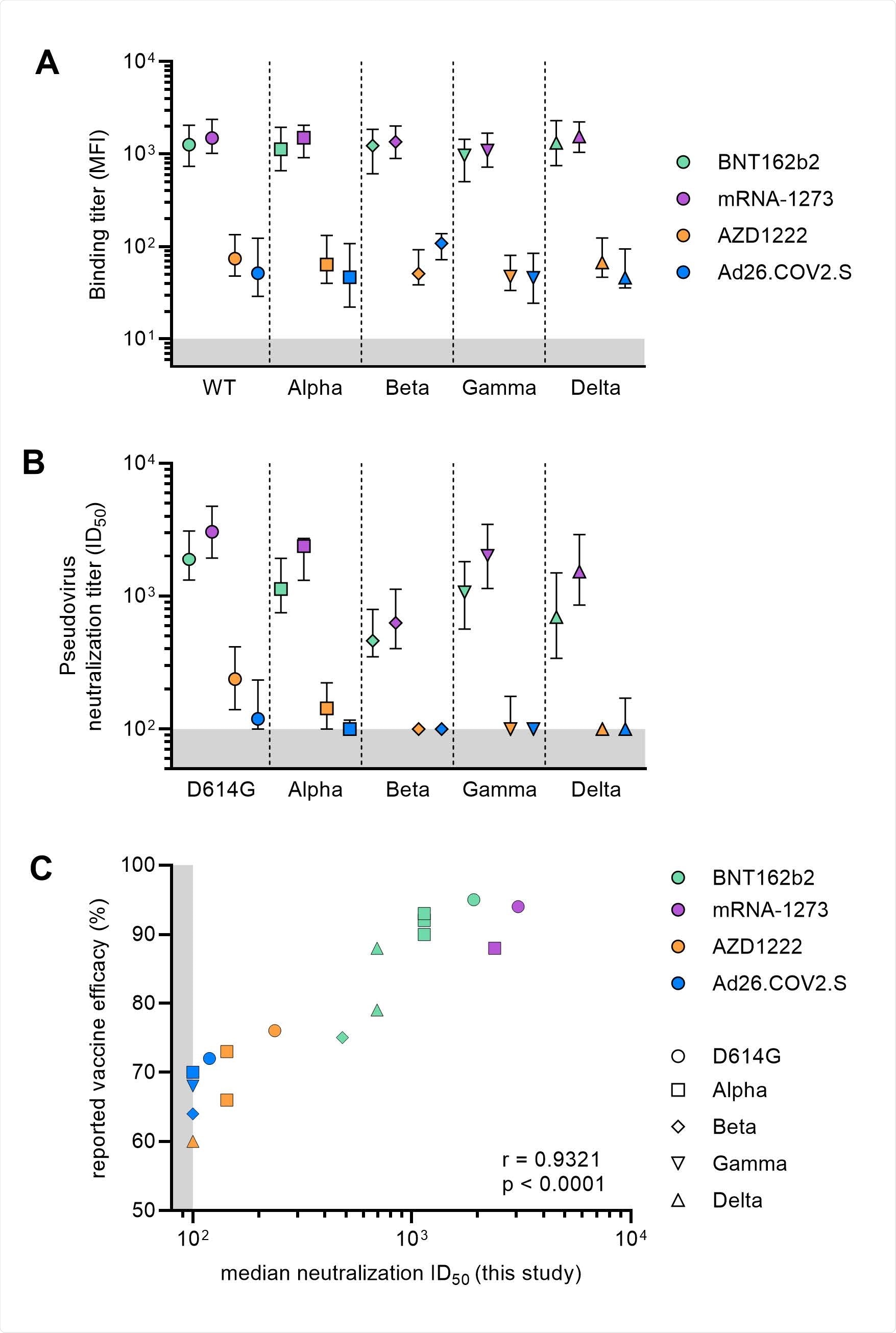
Using a lentiviral-based pseudovirus, the researchers studied vaccine antibody responses against SARS-CoV-2 variants.
For the B.1 variant, neutralizing antibody responses were highest with the Pfizer-BioNTech and the Moderna vaccines. But the Johnson & Johnson and AstraZeneca vaccines had lower neutralizing activity against the variant.
Antibody response after partial vaccination
The researchers next look at antibody responses after one vaccination compared to the single-dose Johnson & Johnson vaccine.
The mRNA vaccines had detectable antibody binding titers against the spike protein after one dose. In contrast, 5 of the 42 individuals taking one-dose AstraZeneca vaccine and 1 of the 13 individuals with the Johnson & Johnson vaccine did not show antibody levels.
A one-dose Moderna vaccine had the highest antibody titers compared to all three vaccines.
Overall, partial vaccination resulted in low neutralizing antibody levels than full vaccination. “The neutralizing antibody levels after one dose were low in all cases (median titers of <100 for BNT162b2 and AZD1222, 119 for Ad26.COV2.S and 300 for mRNA-1273) with only 19 of 45 (42%) BNT162b2, 13 of 35 (37%) AZD1222, 7 of 13 (54%) Ad26.COV2.S and 26 of 31 (84%) mRNA-1273 having detectable neutralization,” explained the researchers.
Antibody levels against variants of concern
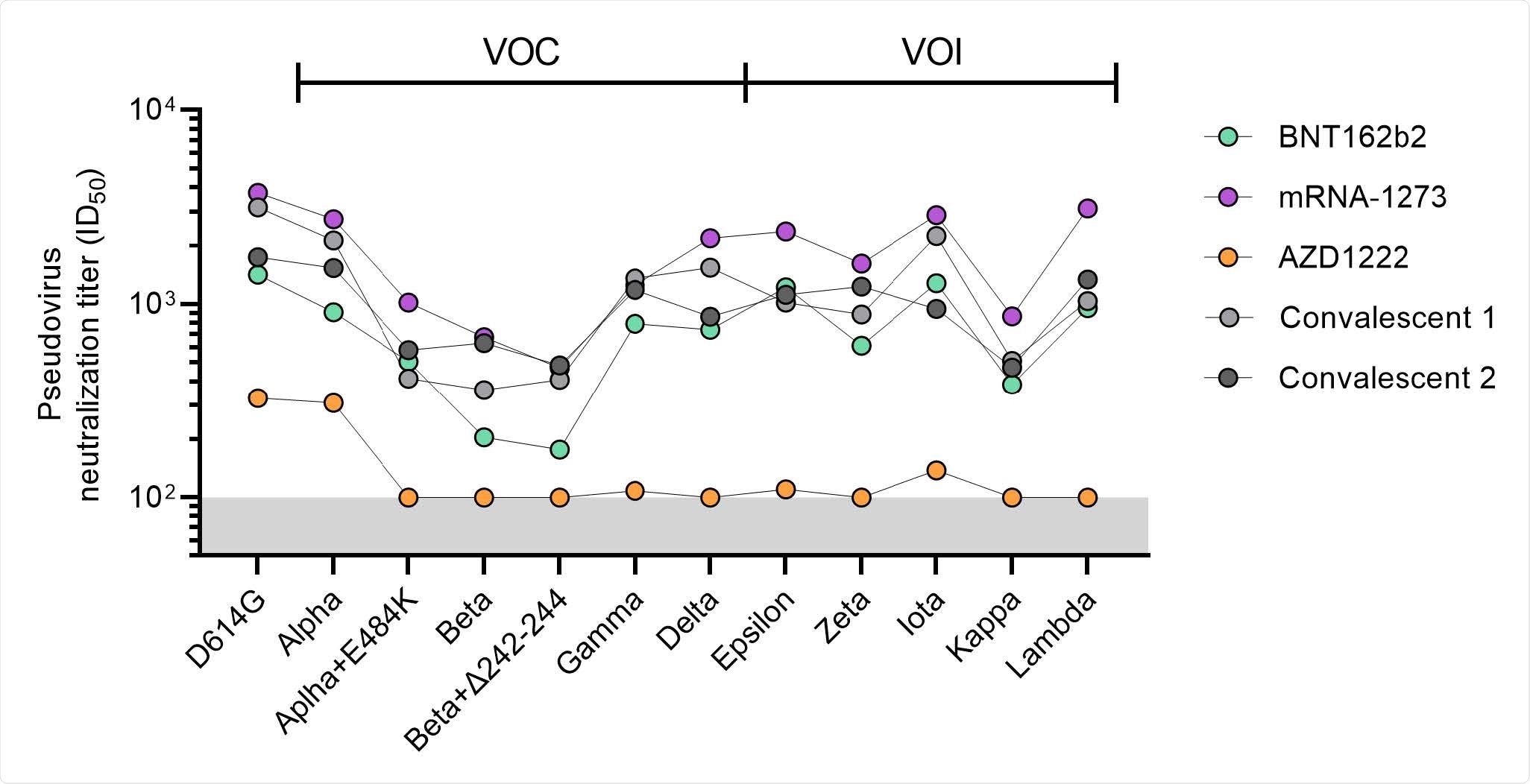
All vaccines showed a drop in antibody levels against SARS-CoV-2 variants, with the most significant reduction in neutralizing activity coming from the Beta variant, followed by Delta, Gamma, and Alpha.
Neutralizing titers against the Alpha, Beta, Gamma, and Delta variants were the highest among individuals with either of the mRNA vaccines.
A good proportion of recipients of the AstraZeneca and Johnson & Johnson vaccines did not show detectable neutralizing activity against SARS-CoV-2 variants.
Neutralization titers were also associated with greater protection from symptomatic COVID-19 infection.
“Overall, the results show that the mRNA vaccines induce substantial levels of neutralizing antibodies against currently defined VOCs, while the adenovirus vector-based vaccines are much less efficient in doing so,” concluded the researchers.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Van Gils MJ, et al. (2021). Four SARS-CoV-2 vaccines induce quantitatively different antibody responses against SARS-CoV-2 variants. medRxiv, 2021. Doi: https://doi.org/10.1101/2021.09.27.21264163, https://www.medrxiv.org/content/10.1101/2021.09.27.21264163v1
Posted in: Medical Research News | Disease/Infection News
Tags: Adenovirus, Antibodies, Antibody, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Fluorescence, Healthcare, Immune Response, immunity, Protein, Pseudovirus, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article
