Computational modeling of therapeutic targets is an important part of modern drug design and discovery. Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, the protein structure of the components of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other relevant molecules such as the angiotensin-converting enzyme 2 (ACE2) receptor have been freely available to researchers via the international Protein Data Bank.
 Study: Characterizing flexibility and mobility in the natural mutations of the SARS-CoV-2 spikes. Image Credit: MedMoMedia / Shutterstock.com
Study: Characterizing flexibility and mobility in the natural mutations of the SARS-CoV-2 spikes. Image Credit: MedMoMedia / Shutterstock.com
Mutations to the SARS-CoV-2 genome that have generated variants of concern include those that alter the three-dimensional (3D) structure of functional SARS-CoV-2 proteins. Precisely identifying these changes and their influence on viral characteristics requires lengthy characterization by methods such as X-ray crystallography, as well as extensive biological assays.
In a paper recently published on the preprint server bioRxiv*, a novel protein modeling method is discussed to assess changes to the conformation of the SARS-CoV-2 spike protein that have been encountered by mutation, which will enable future optimization of drug screening.
SARS-CoV-2 spike protein structures
The structures of the wildtype SARS-CoV-2 spike protein in mid, open, and closed conformations were gathered by the researchers of the current study. The researchers also obtained the structures of the Alpha, Beta, and Gamma variants of concern and the variant identified amongst Mink in 2020.
In total, over 300 structures that represent sub-strains of these variants were collected. Additionally, the structure of each was divided into rigid and flexible parts that could be easily modeled. This allowed several thousand potential conformers to be generated for each protein without the extensive computing required to model each atom individually. Ultimately, the researchers were able to obtain structures that generate steric clash excluded from the final list of possible motions.
The root-mean-square deviation (RMSD) of atomic positions was determined for each protein structure. Herein, the group observed a central rigid cluster that dominates the trimer structure of the SARS-CoV-2 spike protein, with the receptor-binding domain region responsible for changing between open and closed conformations being much more flexible.
The closed spike structures exhibited lower RMSD as compared to that of the open structures. Notably, the open structures amongst all variants of concern had very similar RMSD of 2.53Å, 2.95Å, 2.1Å and 2.28Å in the wildtype, Alpha, Beta, Gamma, and mink variants, respectively. The largest average RMSD in each group was 18.02Å, 22.99Å, 15.44Å, and 17.67Å, respectively.
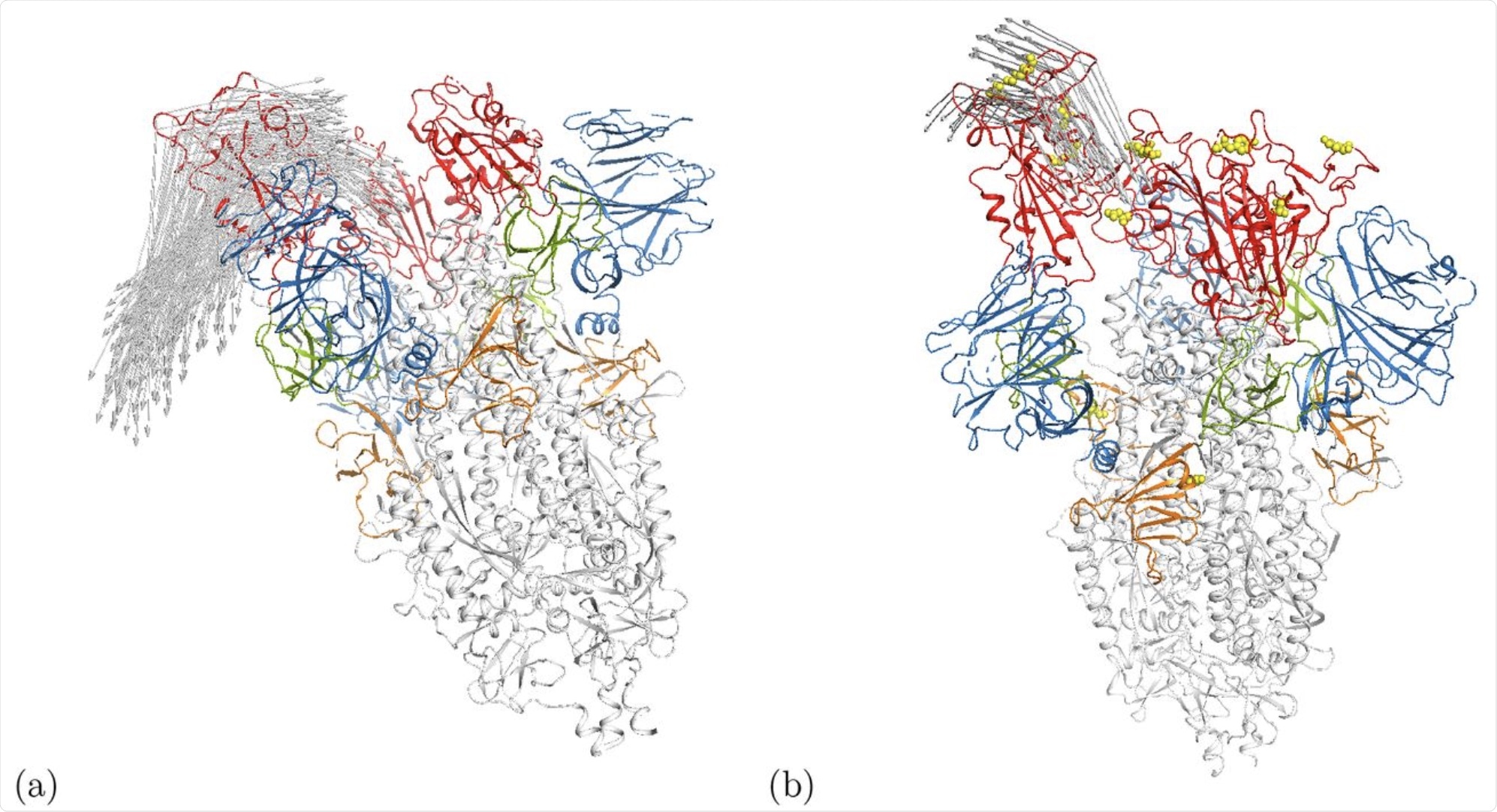 Cartoon representation of motion along modes m7 at Ecut = 1 kcal/mol in the (a) open wild-type spike ecto domain (6VYB) [5] and (b) the γ-variant 7LWW. Arrows indicate motion distances larger than 17.5Å. The RBDs are shown in red while the positions of mutations for 7LWW are given by the yellow spheres. Some other domains are also highlighted in color, i.e. the NTD is blue. An interactive animation of the motion is available for 6VYB [24] and 7LWW [25].
Cartoon representation of motion along modes m7 at Ecut = 1 kcal/mol in the (a) open wild-type spike ecto domain (6VYB) [5] and (b) the γ-variant 7LWW. Arrows indicate motion distances larger than 17.5Å. The RBDs are shown in red while the positions of mutations for 7LWW are given by the yellow spheres. Some other domains are also highlighted in color, i.e. the NTD is blue. An interactive animation of the motion is available for 6VYB [24] and 7LWW [25].
Interestingly, the Gamma variant of SARS-CoV-2 demonstrated motion in the N-terminal domain. In the wildtype and other variants, this region was shown to be somewhat flexible, but the motion was only demonstrated in the gamma variant.
The authors do not determine which mutation may be responsible for the enhanced mobility observed in this region. However, this could be associated with the enhanced immune evasion against convalescent plasma seen in this variant.
Conclusions
Mutations to the SARS-CoV-2 spike protein present in variants of concern are mainly localized to changes in a few residues. Thus, such isolated mutations do not appear to dramatically change the overall flexibility or mobility of the protein.
This is reassuring in terms of future vaccine development, as the overall geometric structure of the spike protein changes little from such mutations. However, many other important factors, such as the charge of the residue, play a role in determining antibody binding affinity. These additional factors were not considered here.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Panayis, J., Romer, N. S., Bellini, D., et al. (2021). Characterizing flexibility and mobility in the natural mutations of the SARS-CoV-2 spikes. bioRxiv. doi:10.1101/2021.09.14.460264. https://www.biorxiv.org/content/10.1101/2021.09.14.460264v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibody, binding affinity, Computational Modeling, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, Crystallography, Enzyme, Genome, Mutation, Pandemic, Protein, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, X-Ray

Written by
Michael Greenwood
Michael graduated from Manchester Metropolitan University with a B.Sc. in Chemistry in 2014, where he majored in organic, inorganic, physical and analytical chemistry. He is currently completing a Ph.D. on the design and production of gold nanoparticles able to act as multimodal anticancer agents, being both drug delivery platforms and radiation dose enhancers.
Source: Read Full Article
