In a recent study posted to the bioRxiv* pre-print server, researchers from Belgium evaluated the efficacy of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antiviral drugs in a severe combined immunodeficient (SCID) mouse model.
 Study: A SCID mouse model to evaluate the efficacy of antivirals against SARS-CoV-2 infection. Image Credit: Kateryna Kon / Shutterstock
Study: A SCID mouse model to evaluate the efficacy of antivirals against SARS-CoV-2 infection. Image Credit: Kateryna Kon / Shutterstock
Background
Due to the incapability of ancestral SARS-CoV-2 strains to bind to the mouse angiotensin-converting enzyme 2 (ACE2) receptor, mouse-adapted (MA) viruses or genetically modified (GM) mice were used to conduct SARS-CoV-2-related preclinical studies.
However, some of the evolved SARS-CoV-2 variants of concern (VOCs), such as the Beta (B.1.351) VOC, bind the murine ACE2 receptor and replicate in some wild-type (wt) mice species, including C57BL/6 and BALB/c mice. The B.1.351 VOC carries spike (S) protein mutations, mainly the N501Y, facilitating efficient murine receptor binding in wt mice. Similarly, a study demonstrated that the K417N mutation was prevalent in a MA SARS-CoV-2 variant.
Together, these findings indicated that both the K417N and E484K mutations in the Beta variant's receptor-binding domain (RBD) facilitate efficient binding to murine ACE2 receptors than SARS-CoV-2 Alpha variants.
About the study
In the present study, researchers used the SCID mouse infection model to study the in vivo efficacy of SARS-CoV-2 antiviral drugs, molnupiravir, and nirmatrelvir. Using a small animal model enabled researchers to test small amounts of antiviral drugs in convenient housing conditions and avoid using the MA SARS-CoV-2 strains and GM mice.
The team intranasally infected nine mice of each wt species with 105 median tissue culture infectious doses (TCID50) of the Beta B.1.351 variant. They euthanized all the mice on day 3 post-infection (pi) to harvest their lungs and quantify the infectious viral titers.
Further, the researchers explored the kinetics of the replication of Beta (B.1.351) VOC in SCID mice. To this end, they infected seven to nine weeks old male SCID mice with 105 TCID50 of the B.1.351 VOC. Between three to seven days pi, the team euthanized 10 test animals and harvested their lungs to quantify the infectious viral titers.
Finally, to assess the potential antiviral efficacy of molnupiravir and nirmatrelvir against the Beta (B.1.351) variant, male SCID mice were treated twice daily with oral doses of either drug or vehicle. In addition, they treated test animals with 200mg/kg of molnupiravir and 300mg/kg of nirmatrelvir for three days starting from the day of infection. The team euthanized antiviral treated mice on day 3 pi for collection of lungs.
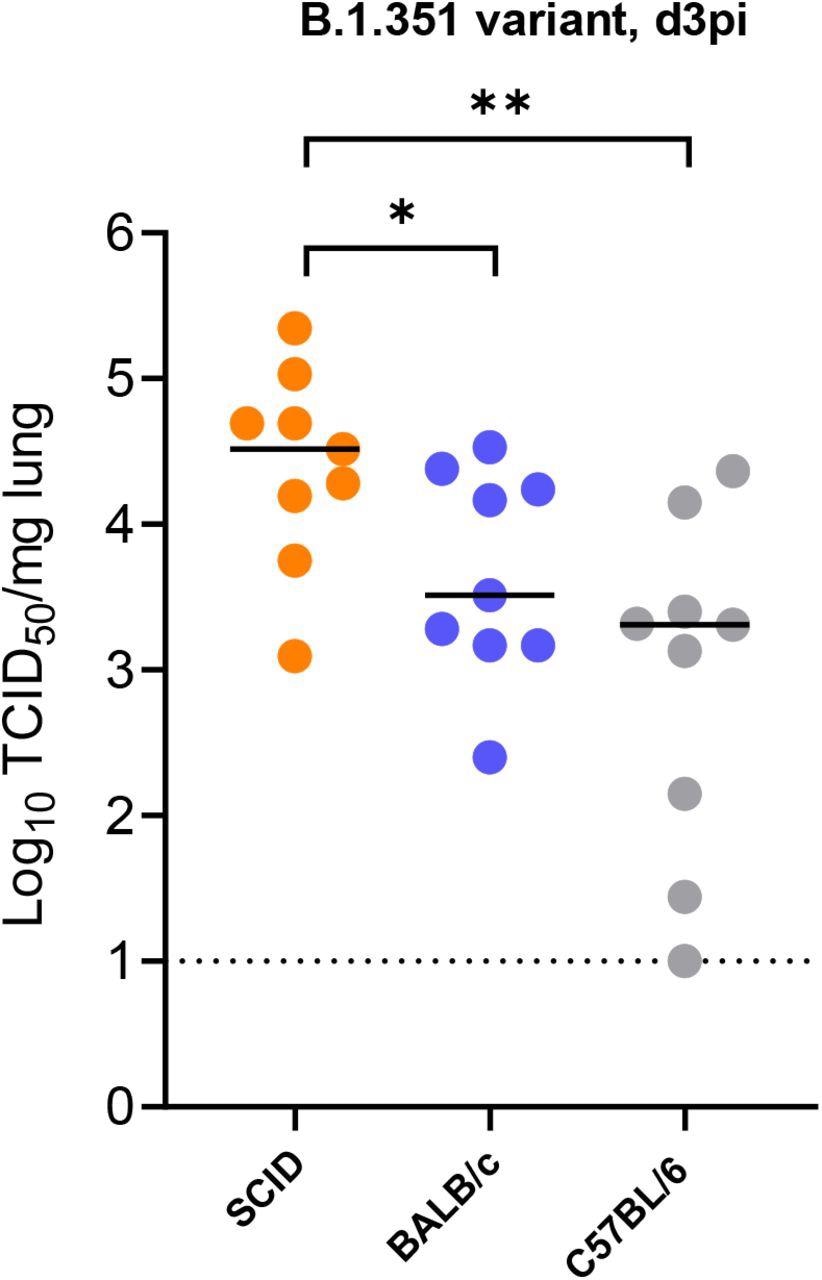
Replication of beta (B.1.351) SARS-CoV-2 in different mice. Infectious viral titers in the lungs of male SCID, male BALB/c and male C57BL/6 mice infected with 105 TCID50 of beta SARS-CoV-2 variants at 3 days post-infection (pi) are expressed as log10 TCID50 per mg lung tissue. Individual data and median values are presented. Data were analyzed with the Mann™Whitney U test. *P < 0.05, **P<0.01). Data are from two independent experiment with n=9 per group.
Study findings
Beta B.1.351 infected male SCID mice had high infectious viral titers and aberrant pathology in the lungs on day 3 pi. The authors noted significantly higher infectious viral titers in the lungs of infected SCID mice than in infected BALB/c and C57BL/6 mice.
From day 4 pi onwards, the infectious virus titers in the lungs began to decline. After day 3 pi, test animals also began to gain weight normally. However, when monitored till day 14 pi, test animals showed no sign of weight loss or morbidity. Histological examinations, however, showed slight peri-bronchial inflammation. Additionally, there was significant perivascular inflammation and intra-alveolar hemorrhage.
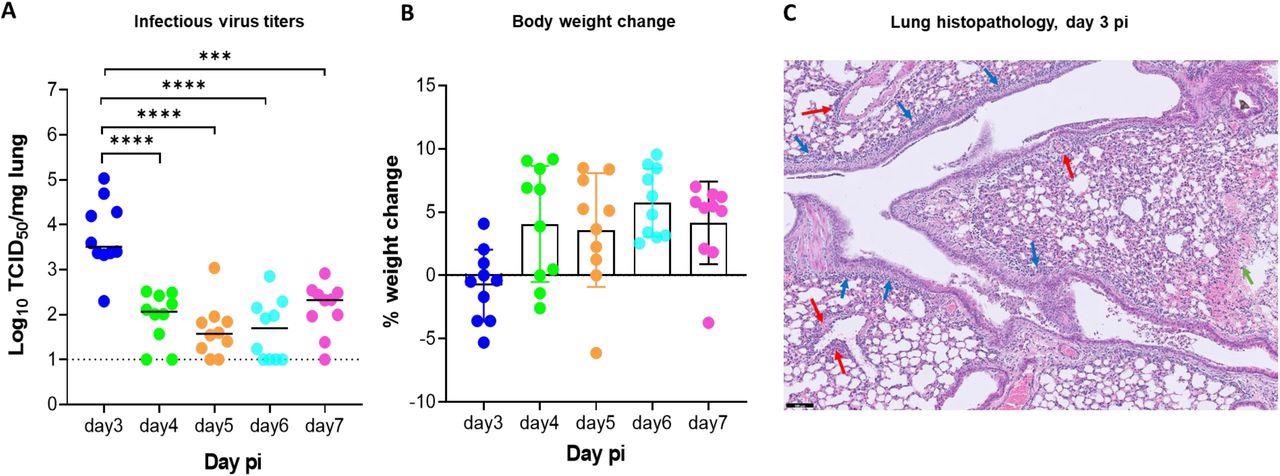
Replication kinetics of beta (B.1.351) SARS-CoV-2 in male SCID mice.(A) Infectious viral loads in the lungs of male SCID mice infected with 105 TCID50 of beta SARS-CoV-2 variants at different days post-infection (pi) are expressed as log10 TCID50 per mg lung tissue. Individual data and median values are presented. Data were analyzed with the Mann™Whitney U test. ***P =0.0003, ****P < 0.0001 (B) Weight change at different days pi in percentage, normalized to the body weight at the time of infection. Bars represent means ± SD. All data are from 2 independent experiments with 10 animals per group. (C) Representative H&E image of lung from SCID mouse infected with the beta variant at day 3 pi showing limited peri-bronchial inflammation (blue arrows), significant peri-vascular inflammation (red arrows) and intra-alveolar hemorrhage (green arrow). Scale Bar=100 µM
Infected mice treated with SARS-CoV-2 antiviral drugs, molnupiravir or nirmatrelvir, showed a substantial dip in the infectious virus titers in their lungs. The molnupiravir and nirmatrelvir-treated groups showed a decline of 1.9 and 3.8 log10 TCID50/mg tissue, respectively, in lung virus titers. Moreover, it significantly improved the lung pathology of the test animals. Notably, four and eight out of 14 animals in the molnupiravir and nirmatrelvir-treated groups, respectively, had no infectious viral titers. As expected, these animals did not lose weight due to the drugs.
Conclusions
The findings of the present study demonstrate the advantages of using SCID mice/ B.1.351 variant infection models for assessing the potential activity of SARS-CoV-2 antivirals in preclinical studies.
The SCID mice infection model was not suitable for the evaluation of vaccines and therapeutic antibodies against SARS-CoV-2. However, they proved beneficial for in vivo efficacy studies of SARS-CoV-2 replication inhibiting drug molecules.
Both molnupiravir and nirmatrelvir have previously been shown to inhibit the replication of the B.1.351 variant in Syrian hamsters. Likewise, they demonstrated high efficacy in the SCID mouse model. Notably, both drugs significantly reduced viral loads in the lung of infected mice with similar potency as they did previously in the Syrian hamster model.
These results are encouraging, especially as molnupiravir, marketed as Lagevrio, is used as an oral drug in several countries for coronavirus disease 2019 (COVID-19) treatment. Nirmatrelvir and ritonavir tablets, marketed as Paxlovid, are also approved for oral use by the United States Food and Drug Administration (FDA) against SARS-CoV-2 and other coronaviruses.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- A SCID mouse model to evaluate the efficacy of antivirals against SARS-CoV-2 infection, Rana Abdelnabi, Caroline S. Foo, Suzanne Kaptein, Robbert Boudewijns, Laura Vangeel, Steven De Jonghe, Dirk Jochmans, Birgit Weynand, Johan Neyts, bioRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.05.13.491916, https://www.biorxiv.org/content/10.1101/2022.05.13.491916v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Animal Model, Antibodies, Coronavirus, Coronavirus Disease COVID-19, covid-19, Drugs, Efficacy, Enzyme, Food, in vivo, Inflammation, Lungs, Mouse Model, Mutation, Pathology, Preclinical, Protein, Receptor, Respiratory, Ritonavir, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Tissue Culture, Vascular, Virus, Weight Loss

Written by
Neha Mathur
Neha is a digital marketing professional based in Gurugram, India. She has a Master’s degree from the University of Rajasthan with a specialization in Biotechnology in 2008. She has experience in pre-clinical research as part of her research project in The Department of Toxicology at the prestigious Central Drug Research Institute (CDRI), Lucknow, India. She also holds a certification in C++ programming.
Source: Read Full Article