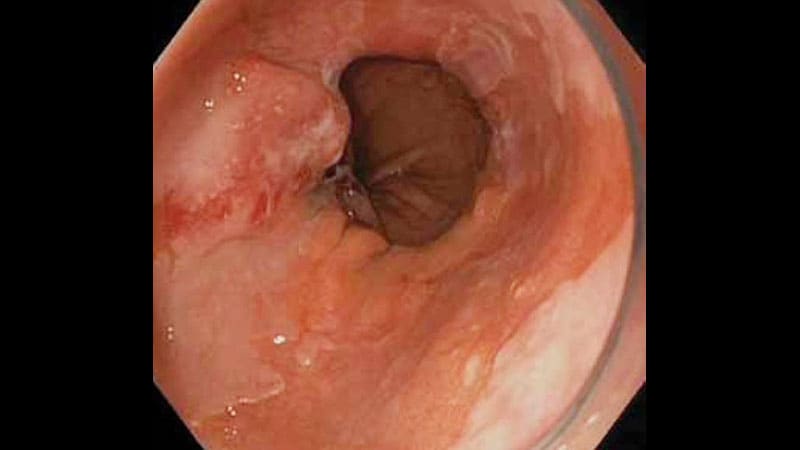
A swallowable, capsule-sponge device used to sample biomarkers offers a reliable, noninvasive alternative to endoscopy for diagnosing Barrett’s esophagus, according to a new guideline from the American College of Gastroenterology.
In addition, the guideline recommends that patients with Barrett’s esophagus with segments of less than 3 cm be screened every 5 years; but if their Barrett’s esophagus segments are 5 cm or greater, they should be screened every 3 years.
“We don’t want to scope everyone for the lowest risk of cancer,” lead author Nicholas J. Shaheen, MD, MPH, chief of gastroenterology and hepatology at the University of North Carolina School of Medicine in Chapel Hill, told Medscape Medical News.
“The traditional way to diagnose Barrett’s esophagus is by upper endoscopy, but it’s expensive and not available everywhere,” he said. “One big change since the last iteration of this guideline is that there’s been a development of nonendoscopic screening modalities.”
The guideline was published in The American Journal of Gastroenterology.
Progress With Swallowable Devices
Solid evidence supports the Cytosponge, a capsule containing a compressed spherical polyurethane sponge attached to a string. The sponge expands to a sphere when the capsule dissolves after being swallowed, Shaheen said.
When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for Barrett’s esophagus, either the protein trefoil factor 3 expressed in intestinal metaplasia or methylated DNA markers associated with Barrett’s esophagus mucosa.
More than 90% of participants in trials have been able to swallow the device. Some mild gagging or throat discomfort has been reported.
In one trial, patients with chronic reflux were randomly assigned to either swallow the Cytosponge or be screened by endoscopy based on the practitioner’s usual care. Any diagnosis with the Cytosponge was confirmed with endoscopy. Barrett’s esophagus was found in 2% of patients who had undergone the Cytosponge procedure, vs less than 1% of those screened in the usual way. Of the 6834 patients in the Cytosponge group, nine were diagnosed with dysplastic Barrett’s esophagus or stage I esophagogastric cancer, vs none of the 6388 participants in the usual-care group.
“At least for now, we’re using the same guidance that we used for endoscopy to decide who might be best served by these devices,” Shaheen said. “There are not good data to make a recommendation yet, but you could easily imagine how you could broaden screening guidelines by having a cheaper, more available test.”
Screening for esophageal cancer may one day be as common as screening for colon cancer, said Herbert C. Wolfsen, MD, a consultant in gastroenterology at the Mayo Clinic in Jacksonville, Florida, and professor of medicine at the Mayo Clinic College of Medicine in Rochester, Minnesota. He was not involved in writing the new guideline.
“We’ve known for years that at least half of patients with esophageal cancer have little, if any, reflux symptoms,” he told Medscape Medical News. “This is an area where the guidelines have been consistent, but I think we’re going to see them start to change.”
Additional Recommendations
The expert panel of the new guideline held off on recommending the use of biomarkers more generally, deeming the evidence insufficient.
The new guideline also endorses endoscopic treatment for dysplastic Barrett’s esophagus but not for Barrett’s esophagus with no dysplasia.
For the first time, it recommends that patients undergo surveillance after successful ablation.
Wolfsen took note of the guideline’s recommendation that clinicians benchmark their practices against published standards.
The guideline mentions “documentation of landmarks and extent of [Barrett’s esophagus], not obtaining biopsies in the setting of a normal-appearing squamocolumnar junction, sampling using the Seattle biopsy protocol, and performing surveillance endoscopy in patients with [nondysplastic Barrett’s esophagus] no sooner than 3–5 years.”
“The acceptance and implementation of these quality metrics is important,” Wolfsen said.
However, the guideline underscores the need for considerably more research, he added.
“This group did a fabulous job,” Wolfsen said. “But when it comes to getting a consensus and people on board, all headed in the same direction, it’s hard when you’re not working with very good data.”
Shahan reports financial relationships with Medtronic, Steris, Pentax, CDx Diagnostics, Interpace Diagnostics, Lucid Medical, Cernostics, Phathom Pharmaceuticals, Exact Sciences, Aqua Medical, and Cook Medical. Wolfsen reports no relevant financial relationships.
Am J Gastroenterol 2022;117:559–587. Full text
Laird Harrison writes about science, health, and culture. His work has appeared in magazines, newspapers, on public radio, and on websites. He is at work on a novel about alternate realities in physics. Harrison teaches writing at the Writers Grotto. Visit him at lairdharrison.com or follow him on Twitter: @LairdH.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article