NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia.
XIIDRA®
This medicine is subject to additional monitoring. This will allow quick identificationof new safety information. You can help by reporting any side effects you may get.You can report side effects to your doctor, or directly at https://www.tga.gov.au/reporting-problems
Lifitegrast 50 mg/mL eye drops, solution
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about XIIDRA.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up-to-date information on the medicine. You can also download the most up-to-date leaflet from www.novartis.com.au
Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you using XIIDRA against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What XIIDRA is used for
XIIDRA is an eye drop containing the medicine lifitegrast.
It is used to treat moderate to severe dry eye disease in adults for whom prior use of artificial tears has not been sufficient.
Lifitegrast is a type of medicine called an ‘LFA-1 antagonist’ (LFA-1 stands for ‘lymphocyte function-associated antigen-1’)
It works by decreasing inflammation in dry eye disease.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
Before you use XIIDRA
When you must not take it
Do not use XIIDRA if you have an allergy to:
any medicine containing lifitegrast
any of the ingredients listed at the end of this leaflet.
Do not take this medicine after the expiry date printed on the carton, foil and single-dose container or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your doctor.
Before you start to use it
XIIDRA should not be used in children and adolescents below 18 years old.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Your doctor can discuss with you the risks and benefits involved.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
If you have not told your doctor about any of the above, tell him/her before you start using XIIDRA.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while using this medicine.
How to use XIIDRA
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box/bottle, ask your doctor or pharmacist for help.
How much to use
The recommended dose is one drop to each affected eye with a single-use container per administration, twice a day.
How to use it
Before you use XIIDRA
Wash your hands before each use. This is to make sure you do not contaminate your eyes.
If you wear contact lenses, remove them before using XIIDRA. You can put your lenses back in 15 minutes after you use XIIDRA.
XIIDRA comes in single-dose containers in an aluminium (foil) pouch. Do not remove the containers from the pouch until you are ready to use XIIDRA.
Do not let the tip of the container touch your eye or any other surfaces. This is to help stop contamination.
About the single-dose containers
Each single-dose container of XIIDRA contains enough for one dose in both of your eyes.
There is some extra solution in the single-dose container. This is in case you miss getting a drop into your eye.
After you have used the drops, throw away the single-dose container and any unused solution.
Do not save any unused XIIDRA.
How to use
Step 1
Take a foil pouch out of the XIIDRA box.
Open the pouch and remove the strip of single-dose containers.
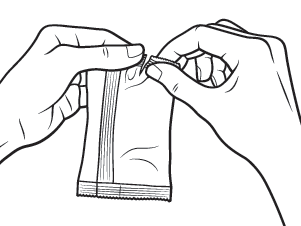
Pull off 1 container from the strip.
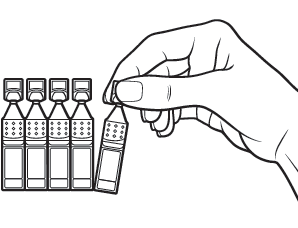
Step 2
Put the remaining strip of containers back in the pouch.
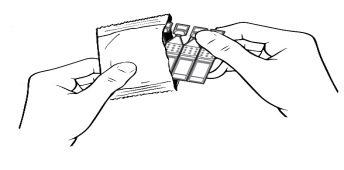
Then fold the edge to close the pouch.
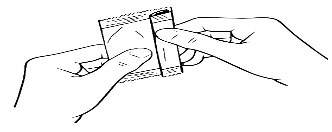
Step 3
Hold the container upright
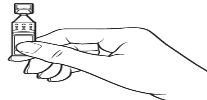
Tap the top of the container until all of the solution is in the bottom part of the container.
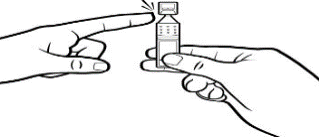
Step 4
Twist off the tab to open the container.
Make sure that the tip of the container does not touch anything. This is to help stop contamination.
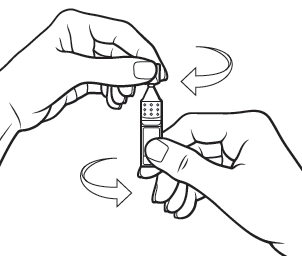
Step 5
Tilt your head backwards.
If you are not able to tilt your head, lie down.
Step 6
Gently pull your lower eyelid downwards, and then look up.
Step 7
Place the tip of the container close to your eye, but be careful not to touch your eye with it.
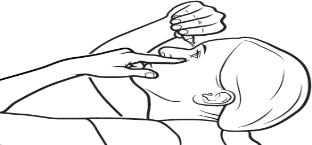
Step 8
Gently squeeze the container.
Let 1 drop fall into the space between your lower eyelid and your eye.
If the drop misses your eye, try again.
Step 9
Repeat steps 5 to 8 for your other eye if required.
There is enough XIIDRA in one container for both eyes.
After use
After you have applied a drop in each affected eye, throw away the opened container, including any remaining solution.
If you use contact lenses, wait for at least 15 minutes before placing them back in your eyes.
How long to use it
You may notice improvements 2 weeks after starting treatment with XIIDRA. It is important that you continue to use XIIDRA every day to get the full benefit of this medicine and to stop signs and symptoms of dry eye disease from returning.
If you forget to use it
If you forget to use XIIDRA, wait until your next dose as planned. Do not use double dose to make up for a forgotten dose.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Do not put any more drops in your eyes. Apply your next dose at the next regular time.
While you are using XIIDRA
Things you must do
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor’s appointments so that your progress can be checked.
Things you must not do
Do not use XIIDRA to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop using your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how XIIDRA affects you.
Your sight may be blurred for a short time after using XIIDRA eye drops. If this happens, wait until you can see clearly before you drive, use any tools or machines.
Side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek medical care immediately if you get any of the following symptoms of an allergic reaction (hypersensitivity) including:
wheezing or difficulty breathing
swollen tongue.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using XIIDRA.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
Eye irritation, pain or discomfort when the drops are used
Temporary unpleasant taste in the mouth
Headache
Blurred vision
Itchy or pink eyes caused by allergic reaction
Increased tears when the drops are used.
Asthma
Discomfort when swallowing
Swelling of the face, lips, mouth or throat.
Skin rash
If you get any side effects that bother you, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
After using XIIDRA
Storage
Keep this medicine in the pack until it is time to use it.
Keep your eye drops in a cool dry place where the temperature stays below 25°C.
After opening the foil pouch, the remaining single-dose containers should be kept in the pouch to protect them from light.
Do not store XIIDRA or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop using this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
XIIDRA is a clear, colourless to slightly coloured solution.
It is supplied in single-dose containers made of low density polyethylene (LDPE). The single-dose containers are packaged in a sealed laminate aluminium pouch.
Ingredients
Active ingredient: lifitegrast. 1 mL of XIIDRA contains 50 mg lifitegrast.
Inactive ingredients:
sodium chloride
sodium phosphate dibasic anhydrous
sodium thiosulfate pentahydrate
sodium hydroxide and/or hydrochloric acid (to adjust pH)
water for injection.
Sponsor
XIIDRA is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1-800-671-203
This leaflet was prepared in April 2020
Australian Registration Number: AUST R: 293589
®= Registered Trademark
(xii040920c.doc) based on PI (xii040920i.doc)
Source: Read Full Article
