In a study published in Nature Neuroscience on Sept. 14, researchers led by Dr. Chen Yelin from the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences showed that the N-methyl-d-aspartic acid (NMDA) receptor (NMDAR) subunit, GluN2A, plays a critical role in mediating the rapid antidepressant effects induced by ketamine.
Importantly, it acts through different mechanisms compared to GluN2B, suggesting that GluN2A could potentially serve as a therapeutic target for fast-acting antidepressants with fewer undesirable side effects.
Depression, which affects approximately 10% of the world’s population, poses significant treatment challenges. Current mainstream antidepressants primarily target the monoaminergic system, such as selective serotonin/norepinephrine reuptake inhibitors (SSRIs/SNRIs). However, these drugs have limitations, including delayed response, ineffectiveness in about 30% of patients, and inability to reduce suicidal risk.
Esketamine (Spravato), the first fast-acting antidepressant approved by the FDA in 2019, has shown rapid and sustained antidepressant effects in treatment-resistant patients. Unfortunately, ketamine treatment can lead to psycho-stimulant side effects and addiction, limiting its broader use. Therefore, it is imperative to elucidate the underlying mechanisms of ketamine to pave the way for the development of safer, fast-acting antidepressants.
In this study, the researchers made groundbreaking discoveries. They found that germline knockout of GluN2A in mice triggered both psycho-mimetic and antidepressant-like behaviors. However, when GluN2A was depleted in adulthood, it induced antidepressant-like responses without causing psycho-mimetic effects. This suggests that targeting GluN2A may offer a novel and potentially safer approach to treat depression.
In addition, they revealed that the rapid antidepressant effects of NMDAR channel blockers such as ketamine and MK-801 were abolished in mice lacking GluN2A. However, these same mice retained the psycho-stimulant effects of MK-801. This indicates that the antidepressant actions and psycho-stimulant effects of ketamine and MK-801 are separable, with GluN2A being specifically required for their antidepressant responses but not for their psycho-stimulant effects.
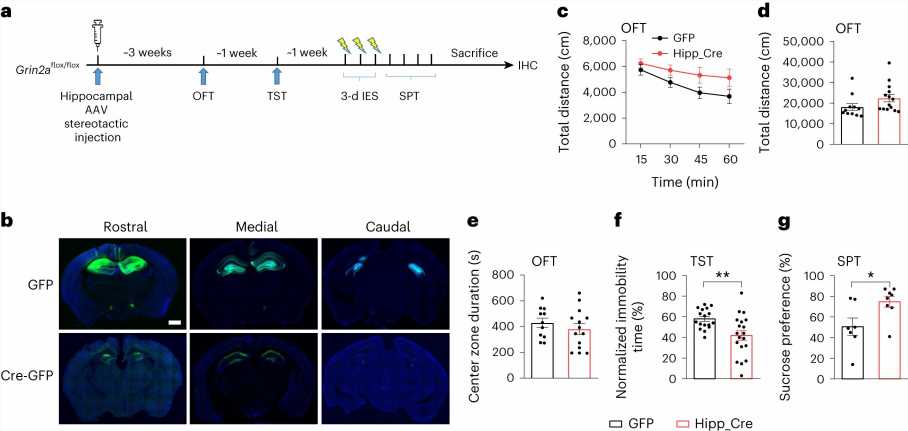
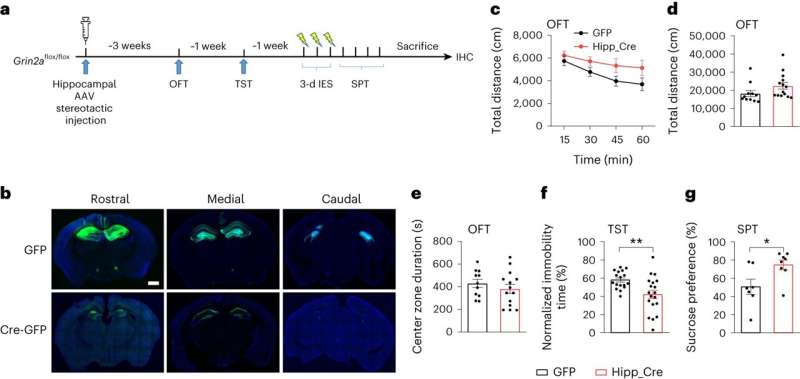
Commonly held beliefs, based on indirect evidence, have suggested that ketamine exerts its antidepressant effects by acting on inhibitory neurons. However, by using the Cre-loxP system to generate animals with conditional depletion of GluN2A in different neuronal subtypes or brain regions, the authors made a crucial discovery.
They found that the knockout of GluN2A in cortical and hippocampal excitatory neurons successfully replicated the spectrum of antidepressant behaviors. Additionally, the removal of GluN2A specifically in hippocampal neurons alone was adequate to trigger antidepressant-like responses.
These findings highlight the critical role of GluN2A in hippocampal excitatory neurons in regulating depressive phenotypes. Significantly, the loss of GluN2A in inhibitory neurons did not induce antidepressant effects. Thus, this study indicates that ketamine induces its antidepressant effects by acting on excitatory neurons, rather than inhibitory neurons.
Further electrophysiological investigations uncovered that the loss of GluN2A significantly increases the intrinsic excitability of hippocampal excitatory neurons. Moreover, the administration of ketamine or MK-801 also rapidly increases the intrinsic excitability of these neurons. Importantly, this increase in neuronal excitability induced by ketamine and MK-801 is dependent on GluN2A, rather than GluN2B.
These findings align closely with the observed behavioral phenotypes, suggesting that GluN2A plays a pivotal role in mediating the antidepressant-like responses induced by ketamine through mechanisms distinct from those of GluN2B.
In conclusion, this study elucidates the critical involvement of GluN2A in the antidepressant responses triggered by ketamine and identifies it as a potential molecular target for the development of safer, fast-acting antidepressants.
More information:
Tonghui Su et al, GluN2A mediates ketamine-induced rapid antidepressant-like responses, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01436-y
Journal information:
Nature Neuroscience
Source: Read Full Article