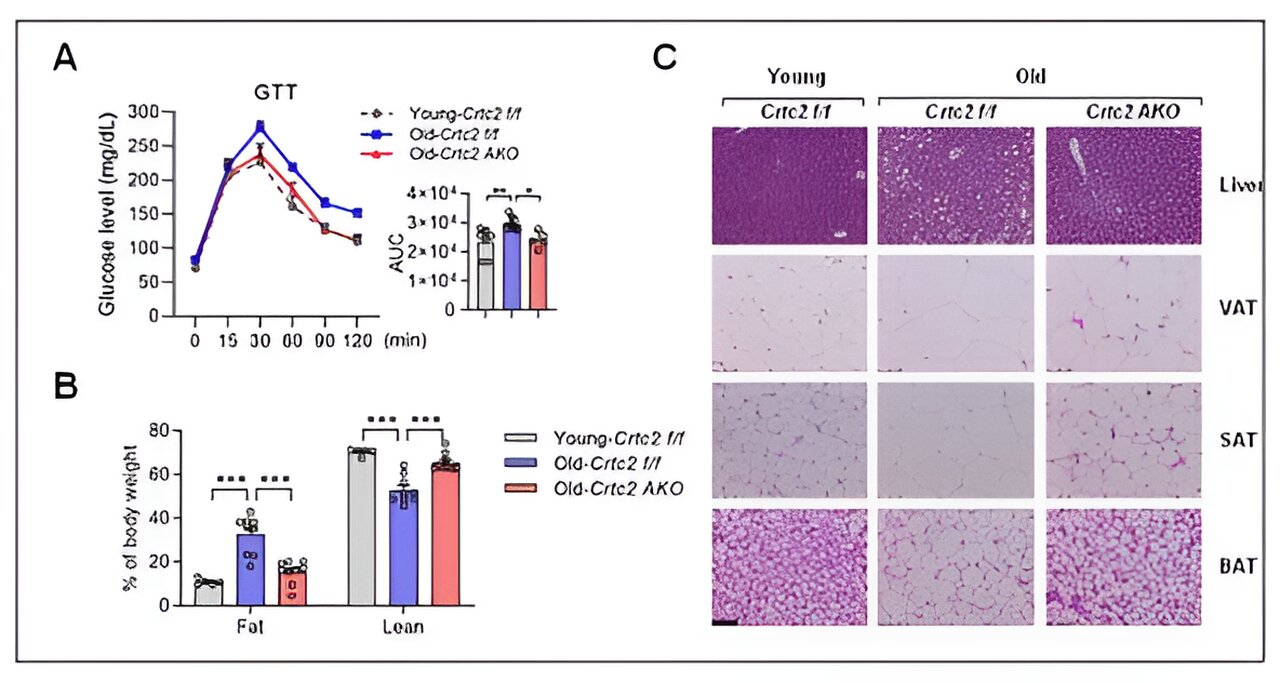
Korean researchers have unveiled a novel signaling pathway that fosters aging-related chronic metabolic disorders.
A research team led by Professor Jong Kyoung Kim from the Department of Life Sciences at POSTECH along with Professor Seung-Hoi Koo from the Division of Life Sciences at Korea University and principal researcher Geum-Sook Hwang from Korea Basic Science Institute (KBSI) have announced the discovery of a new mechanism where BCAA metabolic pathway becomes impaired due to aging, resulting in dysfunctions of adipose cells and chronic metabolic disorders. The research findings were published in Nature Aging on July 24.
Adipocytes play a crucial role in controlling energy metabolic homeostasis. These cells along with preadipocyte cells and various immune cells comprising adipose tissues undergo cellular senescence. The release of the senescence associated secretory phenotype (SASP) from these cells accelerates aging and diminishes the functions of adipose tissues. Consequently, fat accumulation occurs in liver and muscle cells, leading to the onset of metabolic disorders and ultimately reducing health span.
In their earlier research published in Nature Communications, the research team led by Professor Seung-Hoi Koo uncovered that over-activation of CRTC2 induces insulin resistance, fatty liver, and obesity. However, until now, no research findings explored the impact of CRTC2 in adipocytes on aging and its related disorders.
-
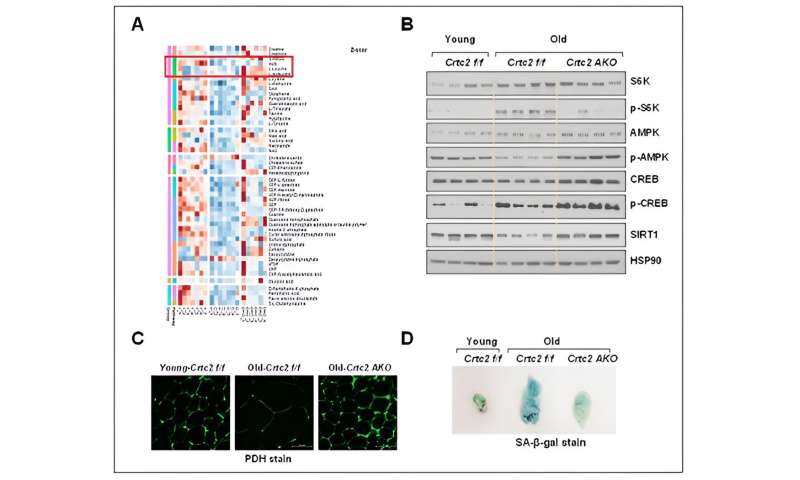
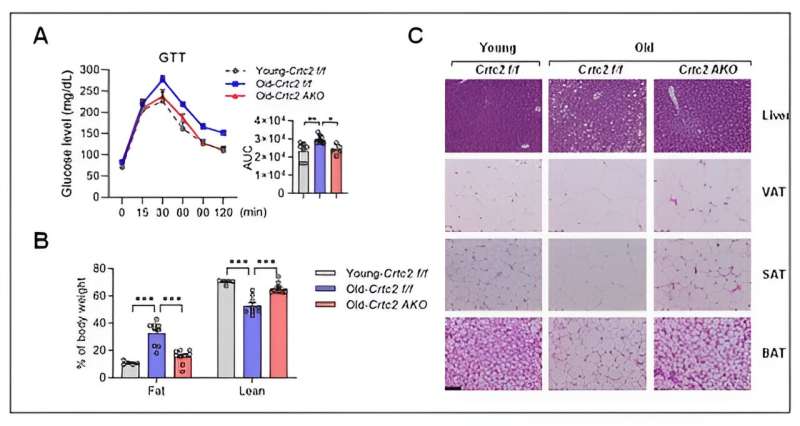
Signaling, mitochondrial functions, and senescence affected by aging and adipocyte-specific Crtc2 knockout. Adipocyte-specific Crtc2 knockout mice (Crtc2 AKO) resulted in a block of decreased BCAA catabolism (A), activation of mTORC1 (B), diminished mitochondrial functions (C), and the adipocyte senescence (D) caused by aging, effectively preventing the generation of aging-related adipocyte dysfunctions. Credit: POSTECH
-
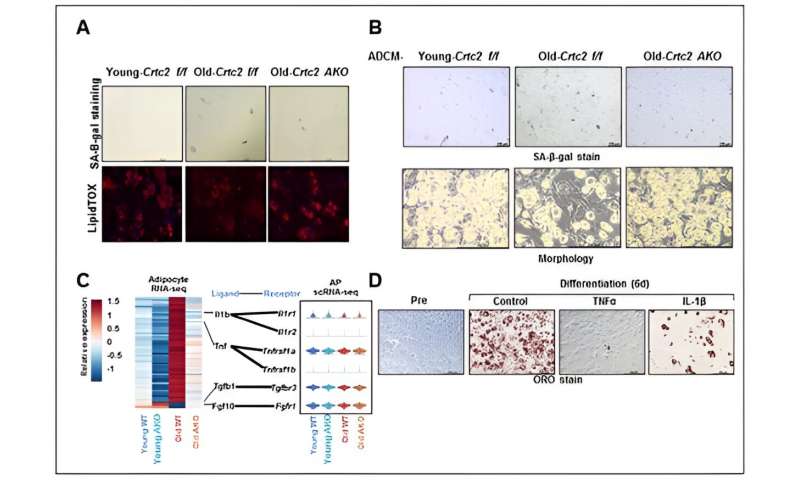
Study of preadipocyte cell differentiation potency affected by aging and adipocyte-specific Crtc2 knockout. Adipocyte-specific Crtc2 knockout mice (Crtc2 AKO) resulted in restoration of adipocyte differentiation potential (A) and a suppression of increased senescence (B). These effects were attributed to a relative reduction in SASP secretion including TNFα and IL-1β that are elevated by aging (C). It was confirmed that SASP effectively inhibits adipocyte differentiation potency (D). Thus, adipocyte-specific Crtc2 knockout effectively suppresses senescence and adipocyte dysfunctions caused by aging. Credit: POSTECH
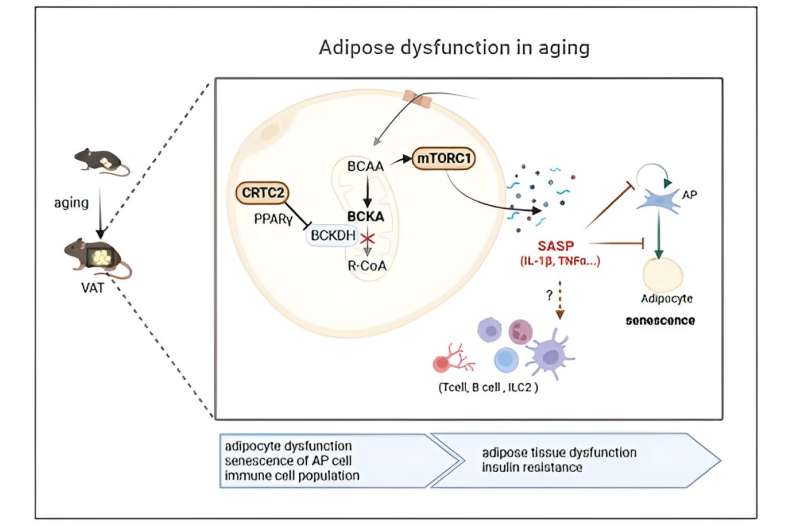
This recent research marks the first confirmation that an increase in adipose CRTC2 due to aging accelerates cellular senescence, leading to a loss of adipocyte functions and aging-related chronic metabolic disorders. CRTC2 reduces the expression of PPAR gamma in adipocytes and impairs catabolism of branched-chain amino acid (BCAA).
Consequently, the mechanistic target of rapamycin complex (mTORC1) becomes activated, as revealed by the composite analysis of metabolome-transcriptome. Increased mTORC1 activation triggers cellular senescence and controls mitochondrial hemostasis, thereby accelerating aging.
The analysis of single-cell transcriptome data showed that aged mice’s adipocytes had increased SASP, particularly IL-1beta and TNF-alpha. This leads to adipose tissue remodeling by inhibiting preadipocyte cell differentiation potency and immunocyte regulations. Notably, mice with CRTC2 removed from their adipocytes displayed limited activation of BCAA-mTORC1 axis, ultimately inhibiting the development of chronic metabolic disorders associated with aging. This suggests that aging can be mitigated by controlling CRTC2 or BCAA catabolism.
Professor Seung-Hoi Koo explained, “This study employed the latest convergent omics technology to unveil, for the first time, that an increase of CRTC2 in adipocytes due to aging leads to impaired BCAA catabolism, which is the primary cause of cell aging and metabolic disorders. Consequently, selective inhibition of CRTC2 or activation of PPAR gamma in adipocytes may hold the potential to inhibit aging and extend health span.”
More information:
Hye-Sook Han et al, Impaired BCAA catabolism in adipose tissues promotes age-associated metabolic derangement, Nature Aging (2023). DOI: 10.1038/s43587-023-00460-8
Journal information:
Nature Communications
,
Nature Aging
Source: Read Full Article