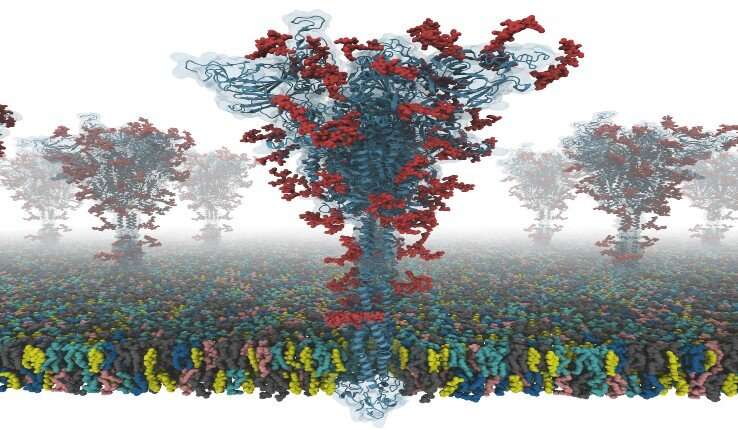
The virus SARS coronavirus 2 (SARS-CoV-2) is the known cause of coronavirus disease 2019 (COVID-19). The “spike” or S protein facilitates viral entry into host cells.
Now a group of researchers from Lehigh and Seoul National University in South Korea and the University of Cambridge in the UK have worked together to produce the first open-source all-atom models of a full-length S protein. The researchers say this is of particular importance because the S protein plays a central role in viral entry into cells, making it a main target for vaccine and antiviral drug development.
This video illustrates how to build the membrane system from their SARS-CoV-2 S protein models. The model-building program is open access and can be found from the home page of CHARMM-GUI at the COVID-19 Archive.
Developed by Wonpil Im, a professor in Lehigh’s Department of Biological Sciences and Bioengineering Department, CHARMM-GUI (graphical user interface) is a program that simulates complex biomolecular systems simply, precisely and quickly. Im describes it as a “computational microscope” that enables scientists to understand molecular-level interactions that cannot be observed any other way.
https://youtube.com/watch?v=1EhOWs-GZvQ%3Fcolor%3Dwhite
“Our models are the first fully-glycosylated full-length SARS-CoV-2 spike (S) protein models that are available to other scientists,” says Im. “I was fortunate to collaborate with Dr. Chaok Seok from Seoul National University in Korea and Dr. Tristan Croll from University of Cambridge in the U.K. Our team spent days and nights to build these models very carefully from the known cryo-EM structure portions. Modeling was very challenging because there were many regions where simple modeling failed to provide high-quality models.”
Scientists can use the models to conduct innovative and novel simulation research for the prevention and treatment of COVID-19, according to Im.
The S protein structure was determined with cryo-EM with the RBD up (PDB ID: 6VSB), and with the RBD down (PDB ID: 6VXX). But this model has many missing residues. So, they first modeled the missing amino acid residues, and then other missing domains. In addition, they modeled all potential glycans (or carbohydrates) attached to the S protein. The glycans prevent antibody recognition, which makes it difficult to develop a vaccine. They also built a viral membrane system of an S protein for molecular dynamics simulation.
Source: Read Full Article
