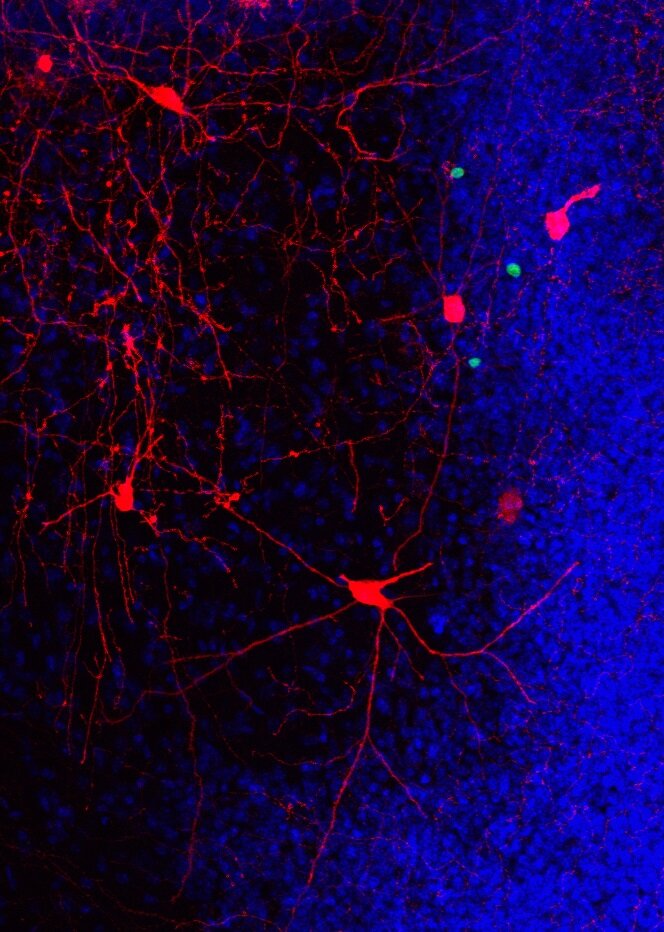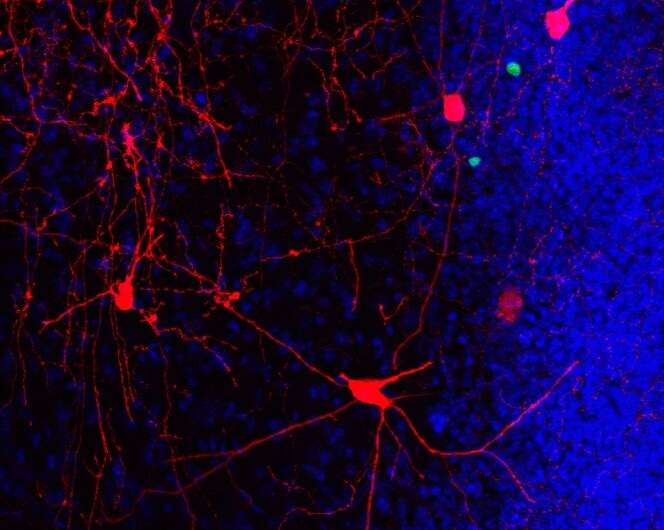
Aging often is accompanied by cognitive decline. Among the first structures of the brain affected are the hippocampus and adjacent cortices, areas essential for learning and memory. Deficits in cognitive ability are associated with reduced hippocampal volume and degradation of synaptic connectivity between the hippocampus and the (peri)-entorhinal cortex.
Increasing evidence indicates that physical activity can delay or prevent these structural and functional reductions in older adults. A new study by Florida Atlantic University and CINVESTAV, Mexico City, Mexico, provides novel insight into the benefits of exercise, which should motivate adults to keep moving throughout their lifetime, especially during middle age.
For the study, researchers focused on the effects of long-term running on a network of new hippocampal neurons that were generated in young adult mice, at middle age. These “mice on the run” demonstrate that running throughout middle age keeps old adult-born neurons wired, which may prevent or delay aging-related memory loss and neurodegeneration.
Adult-born neurons are thought to contribute to hippocampus-dependent memory function and are believed to be temporarily important, during the so-called ‘critical period’ at about three to six weeks of cell age, when they can fleetingly display increased synaptic plasticity. However, these new neurons do remain present for many months, but it was unclear whether those born in early adulthood remain integrated into neural networks and whether their circuitry is modifiable by physical activity in middle age.
To address these questions, researchers used a unique rabies virus-based circuit tracing approach with a long-time interval between the initial labeling of new neurons and subsequent analysis of their neural circuitry in rodents. More than six months after tagging of the adult-born neurons with a fluorescent reporter vector, they identified and quantified the direct afferent inputs to these adult-born neurons within the hippocampus and (sub)cortical areas, when the mice were middle-aged.
Results of the study, published in the journal eNeuro, show long-term running wires ‘old’ new neurons, born during early adulthood, into a network that is relevant to the maintenance of episodic memory encoding during aging.
“Long-term exercise profoundly benefits the aging brain and may prevent aging-related memory function decline by increasing the survival and modifying the network of the adult-born neurons born during early adulthood, and thereby facilitating their participation in cognitive processes,” said Henriette van Praag, Ph.D., corresponding author, an associate professor of biomedical science in FAU’s Schmidt College of Medicine and a member of the FAU Stiles-Nicholson Brain Institute.
Findings from the study showed long-term running significantly increased the number of adult-born neurons and enhanced the recruitment of presynaptic (sub)-cortical cells to their network.
“Long-term running may enhance pattern separation ability, our ability to distinguish between highly similar events and stimuli, a behavior closely linked to adult neurogenesis, which is among the first to display deficits indicative of age-related memory decline,” said Carmen Vivar, Ph.D., corresponding author, Department of Physiology, Biophysics and Neuroscience, Centro de Investigacion y de Estudios Avanzados del IPN in Mexico.
Aging-related memory function decline is associated with the degradation of synaptic inputs from the perirhinal and entorhinal cortex onto the hippocampus, brain areas that are essential for pattern separation, and contextual and spatial memory.
“We show that running also substantially increases the back-projection from the dorsal subiculum onto old adult-born granule cells,” said van Praag. “This connectivity may provide navigation-associated information and mediate the long-term running-induced improvement in spatial memory function.”
Results from the study show that running not only rescued perirhinal connectivity but also increased and altered the contribution of the entorhinal cortices to the network of old adult-born neurons.
“Our study provides insight as to how chronic exercise, beginning in young adulthood and continuing throughout middle age, helps maintain memory function during aging, emphasizing the relevance of including exercise in our daily lives,” said Vivar.
More information:
Carmen Vivar et al, Running throughout Middle-Age Keeps Old Adult-Born Neurons Wired, eNeuro (2023). DOI: 10.1523/ENEURO.0084-23.2023
Journal information:
eNeuro
Source: Read Full Article