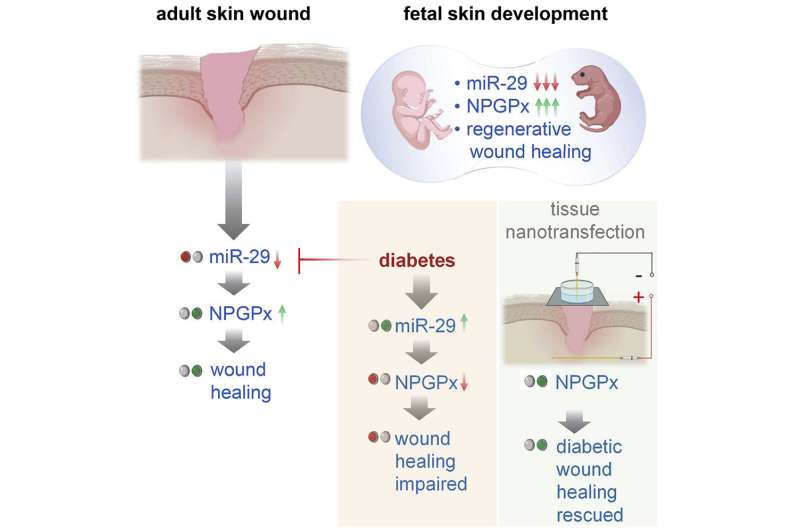
Researchers at Indiana University School of Medicine are looking for ways to heal wounds by using a healing protein that is active in fetuses, but largely inactive in adults and absent in diabetic adults.
“We already know from previous studies at other institutions that if a fetus is wounded, it can regenerate the tissue, or repair it to be like new,” said Chandan K. Sen, Ph.D., associate vice president of military and applied research, the J. Stanley Battersby chair and distinguished professor of surgery and director of the Indiana Center for Regenerative Medicine and Engineering at Indiana University School of Medicine.
“But after birth, such regenerative wound healing ability is lost. Healing in adults is relatively inefficient [and] often associated with undesirable scar formation.”
In the study, published recently in Molecular Therapy, the team focused on a protein called nonselenocysteine-containing phospholipid hydroperoxide glutathione peroxidase, or NPGPx. NPGPx is active in fetal tissue but becomes mostly inactive in the skin after birth.
“Nature essentially hides this fetal regenerative repair pathway in the adult body,” Sen said. “We spotted its absence, and then activated it to improve healing of diabetic wounds.”
Researchers used tissue nanotransfection technology developed by faculty at the ICRME to deliver the NPGPx gene to the wound site. Diabetic wounds, which are complicated skin injuries in people with diabetes, are particularly difficult to treat and often lead to amputations or other complications because of how easily they can become infected.
“This is an exciting new approach to harness fetal repair mechanisms to close diabetic wounds in adults,” Sen said.
“The study results show that while NPGPx has been known to be abundant in the fetal skin, but not after birth, it can be reactivated in the skin after an injury. We look forward to continued study aiming to achieve a more complete regenerative repair by improving our understanding of how NPGPx functions.”
More information:
Subhadip Ghatak et al, Driving adult tissue repair via re-engagement of a pathway required for fetal healing, Molecular Therapy (2022). DOI: 10.1016/j.ymthe.2022.09.002
Journal information:
Molecular Therapy
Source: Read Full Article
