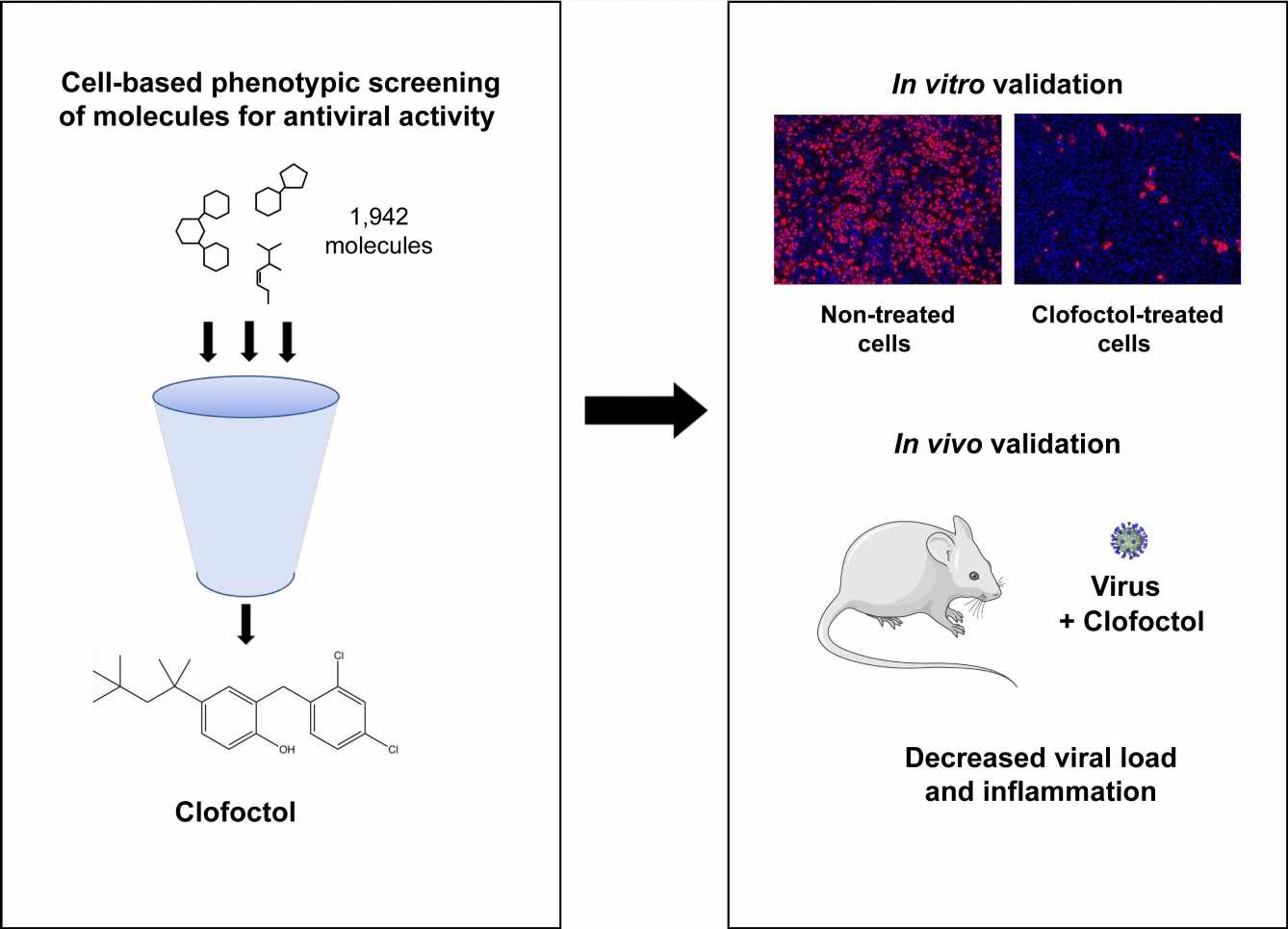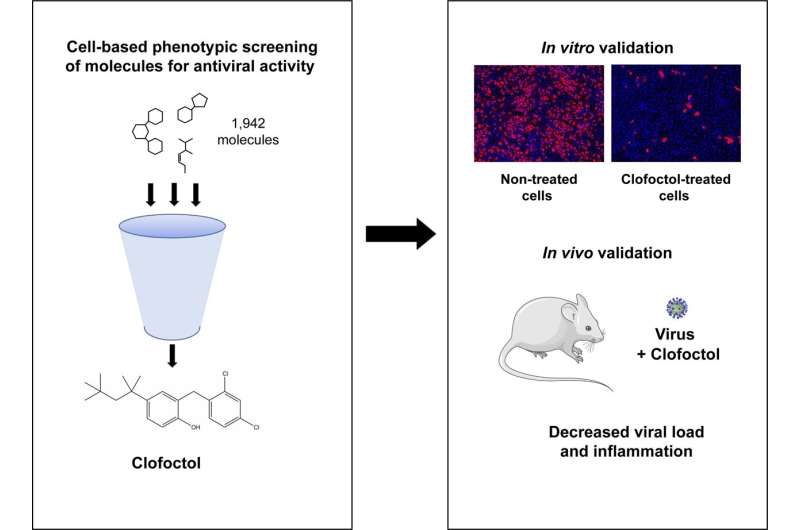
Repurposed drugs may have a speedier path to clinical use because they have already been shown to be safe in people. A study publishing May 19 in the open access journal PLOS Pathogens by Sandrine Belouzard and Jean Dubuisson at Pasteur Institute, Lille, France, and colleagues suggests clofoctol may be an effective treatment for SARS-CoV-2 infections in mice.
While COVID-19 vaccines reduce hospitalizations and death, they do not control virus transmission, and affordable, effective therapies are needed. Previous attempts to repurpose medicines to treat COVID-19 patients have been unsuccessful. In order to identify potential antiviral therapies effective against COVID-19, authors accessed the Apteeus drug library, a collection of 1,942 approved drugs to identify molecules that exhibit antiviral activity against SARS-CoV-2. The authors selected clofoctol based on its antiviral potency and tested their hypothesis by testing its effects in SARS-CoV-2-infected mice.
The researchers found that transgenic mice treated with clofoctol had a decreased viral load, reduced inflammatory gene expression, and lowered pulmonary pathology. Future studies are needed to further understand the drug’s therapeutic potential in SARS-CoV-2 patients as the study was limited by the physiological differences between humans and mice. Additionally, the mice were sacrificed only two days after treatment, so longer-term effects remain unknown.
According to the authors, “The antiviral and anti-inflammatory properties of clofoctol, associated with its safety profile and unique pharmacokinetics make a strong case for proposing clofoctol as an affordable therapeutic candidate for the treatment of COVID-19 patients. Finally, the relatively low cost of this drug suggests that it is a potential clinical option for treatment of COVID-19 patients in resource-poor settings.”
Source: Read Full Article