Predictions are becoming more and more a part of our lives, and they are becoming increasingly useful in medical science as the science evolves. Increased understanding of disease and its treatments allows us to use predictions based on predictive biomarker signatures to optimize treatment outcomes for increasingly specific subject groups.
Developing prediction-driven decision rules in the form of pooled testing methods for HIV treatment failure, identifying randomized controlled trial (RCT) designs capable of rigorously evaluating these prediction-driven decision rules, and studying survival analysis methods capable of analyzing the data from such RCTs, whether proportional hazards holds or not, are the subjects of a new dissertation by Adam Brand, Ph.D. student at the Department of Medical Epidemiology and Biostatistics. His studies include five projects (three published works and two manuscripts) that at first glance may seem disjointed, but they followed the natural thought process of always asking the question “What next?”
In the first project, Adam developed novel prediction-based decisions rules for treatment management of HIV patients. In the second project he identified the proper RCT design to test the effectiveness of their prediction-driven decision rules. In projects 3-5 he studied ways of analyzing the survival data that would be the outcome of their prediction-driven RCT. Adam developed new implementations for R of RMST-based statistical inference methods and compared those methods to the benchmark methods of testing for differences in survival between two groups in realistic group sequential RCT settings.
What are the most important conclusions in your thesis?
“Throughout my work, we discovered what we viewed as holes or inaccuracies in the current research, and we thought it important to fill those holes and correct those inaccuracies. I feel that we have developed promising prediction-driven decision rules for HIV treatment management, and that we have developed a deep understanding on how to rigorously test those rules, hoping we contributed to statistical knowledge and understanding along the way.”
What do you think should be done moving forward?
“I would like to see a confirmatory randomized trial conducted in the resource-limited regions for which these pooled testing methods are intended. If two such trials show increased efficiency of these pooled testing methods without harming the safety of the patients, the methods should be implemented, hopefully allowing more HIV infected patients to receive effective, regular testing for HIV treatment.”
More information:
Adam Brand et al, Prediction‐driven pooled testing methods: Application to HIV treatment monitoring in Rakai, Uganda, Statistics in Medicine (2021). DOI: 10.1002/sim.9022
Adam Brand et al, Confirmatory prediction-driven RCTs in comparative effectiveness settings for cancer treatment, British Journal of Cancer (2023). DOI: 10.1038/s41416-023-02144-x
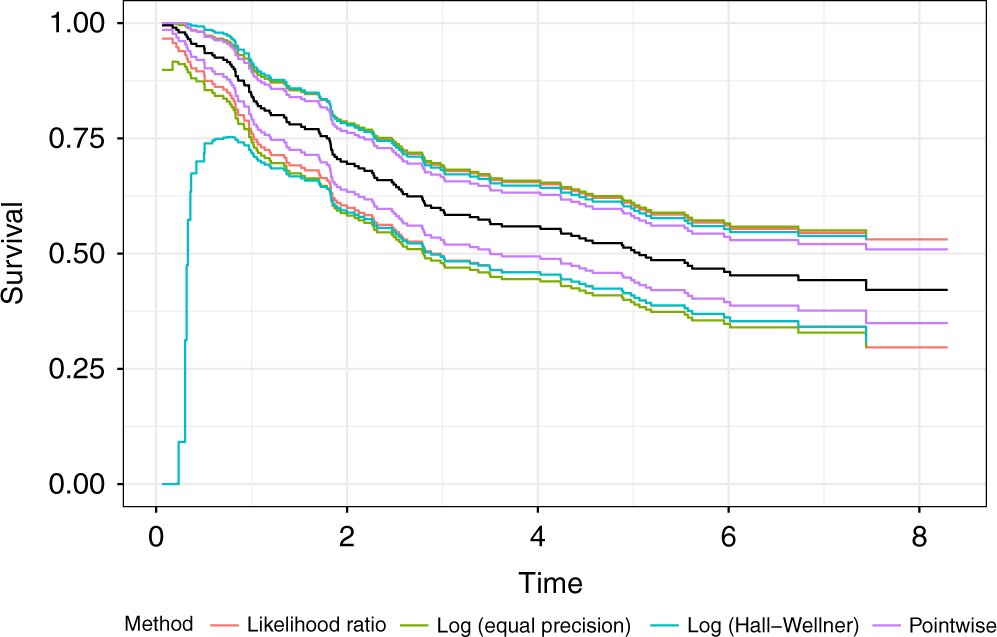
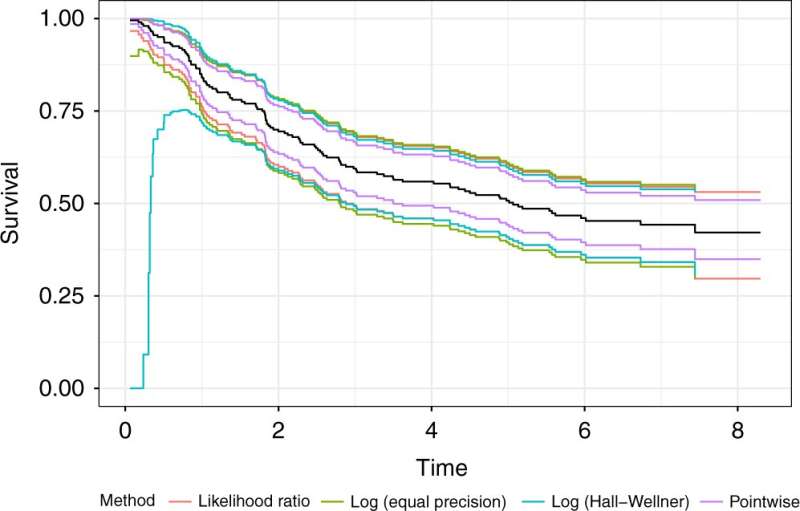
Michael C. Sachs et al, Confidence bands in survival analysis, British Journal of Cancer (2022). DOI: 10.1038/s41416-022-01920-5
Brand A, Sachs MC, Gabriel EE. Estimating differences in restricted mean survival time in R with two new implementations. [Manuscript]
Brand A, Sachs MC, Gabriel EE. Evaluating restricted mean survival time methods in group sequential RCTs. [Manuscript]
Journal information:
British Journal of Cancer
Source: Read Full Article