A research collaboration between Monash University and Lava Therapeutics details a novel immune-oncology approach for the potential treatment of cancer. Instrumental to the study was co-first author Dr. Roeland Lameris from Amsterdam UMC and colleagues from the University of Melbourne.
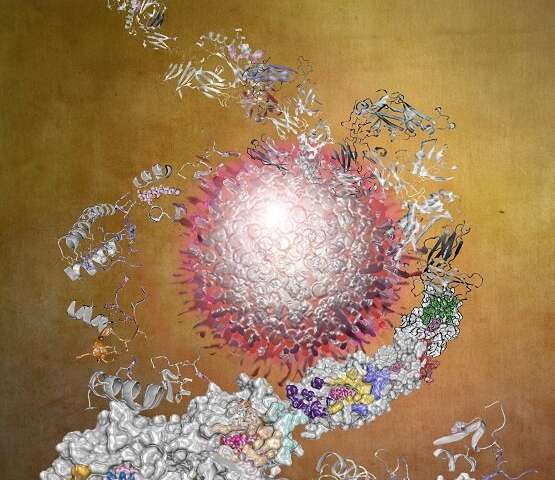
Published in Nature Cancer, the study, co-led by Monash Biomedicine Discovery Institute’s ARC Laureate Fellow Professor Jamie Rossjohn and Dr. Adam Shahine, highlights the synergy between an antibody fragment, known as a nanobody, that not only acts as a bridge helping to link together two key immune cell receptors but also takes advantage of their interaction, enabling the body to enhance its immune response to cancer.
These antibody fragments, denoted as nanobodies, act by targeting the interaction between a molecule known as CD1d and Natural Killer T cells (NKT) in a stable and long-lasting manner, against tumor samples of patients with multiple myeloma and acute myeloid leukemia.
The new findings will serve as a model for the potential generation of new and effective therapies against a broad range of cancers.
Using the Australian Synchrotron, the team at Monash University provided detailed atomic insight into how the nanobodies exerted their effect on immune cells in a cancer model. “We were able to precisely visualize how the nanobody simultaneously recognized CD1d and the NKT TCR, thereby providing a molecular basis for their anti-tumor properties,” Professor Rossjohn stated.
Hans van der Vliet, professor in medical oncology at Amsterdam UMC and chief scientific officer of Lava Therapeutics, says “By targeting and boosting natural immune cells that are inherent in all humans, such as NKT cells and gamma-delta T cells, for an enhanced therapeutic effect, we believe our approach could ultimately translate into a broadly applicable immunotherapeutic approach for a range of cancer indications.”
Source: Read Full Article
