Several vaccines have been developed against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to curb the ongoing coronavirus disease 2019 (COVID-19) pandemic. As of December 28, 2021, the United States Centers for Disease Control and Prevention (CDC) have reported at least 77.6% of the U.S. population over the age of five have received at least one dose of the COVID-19 vaccine.
A new research letter published in JAMA Network Open evaluates whether the estimated vaccine effectiveness changes against infection over time in an effort to help inform public health policy and clinical practices.
Study: Incidence and Estimated Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Among Persons Tested in US Retail Locations, May 1 to August 7, 2021. Image Credit: vovidzha / Shutterstock.com
About the study
The current case-control study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The study used a national SARS-CoV-2 database to evaluate unique patients who were symptomatic for SARS-CoV-2 infection.
Positive SARS-CoV-2 infections were confirmed using polymerase chain reaction (PCR) tests. Data for the study were collected from May 1, 2021, to August 7, 2021.
The study participants answered a screening questionnaire that included questions regarding COVID-19–related symptoms per CDC definition, vaccination status, number of doses, and vaccination time before collection of nasal swabs. BNT162b2 (Pfizer), mRNA-1273 (Moderna), and JNJ-78436735 (Johnson & Johnson) vaccines were used for analysis. SARS-CoV-2 negative cases were used as controls to determine the effectiveness of the vaccines.
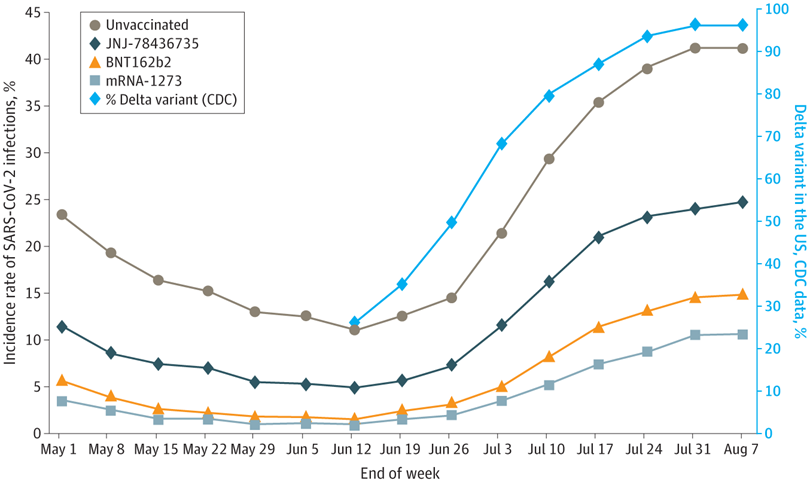
Persons had at least 1 self-reported symptom per US Centers for Disease Control and Prevention (CDC) definition (fever, shortness of breath, cough, chills, nausea or vomiting, muscle pain, sore throat, loss of taste or smell, fatigue, headache, congestion, diarrhea). Vaccinated persons included all receiving any number of doses of any of the 3 vaccines (for mRNA vaccines, 99.9% of persons reported receiving 2 doses). Data on Delta virus proportions were obtained from the CDC website.
Study findings
The results indicated that out of the 1,237,097 individuals included in the study, 59.2% were women, 40.7% were men, and 0.1% were unknown. The study also involved diverse groups that included Asians, Black or African Americans, White, Hispanic, Alaska Natives, Pacific Islanders, and American Indian individuals. Of the vaccinated individuals, 27.1% received the BNT162b2 vaccine, 16.8% received the mRNA-1273 vaccine, and 4% received the JNJ-78436735 vaccine.
The results reported that individuals who received the messenger ribonucleic acid (mRNA) vaccines had the lowest incidence rate, while unvaccinated individuals had the highest. The unvaccinated individuals were found to have 412%, 287%, and 159% more infections as compared to those who had received the mRNA1273, BNT162b2, or JNJ-78436735 vaccines, respectively.
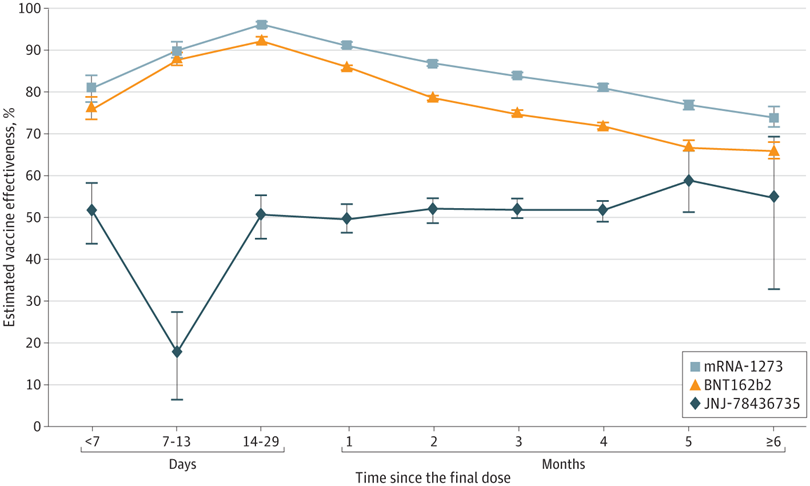
Models were adjusted for age, geographic region, and calendar month of test. Estimated vaccine effectiveness over time since the final dose was calculated as (1 − multivariable-adjusted odds ratio for infection) × 100. The final dose was defined as the only dose for JNJ-78436735 and the second dose for mRNA-1273 or BNT162b2 before August 7, 2021.
The incidence rate was observed to be 24.8% for unvaccinated individuals, 15.6% for those receiving the JNJ-78436735 vaccine, 8.6% for the BNT162b2 vaccine, and 6% for the mRNA1273 vaccine. The magnitude of SARS-CoV-2 infection was observed to be even higher for unvaccinated individuals during the prevalence of the Delta strain.
The vaccine effectiveness for those who had received two doses of the mRNA vaccines peaked after two weeks then fell to 86.8% in two to three months and further to 74.2% after six months. For one dose of JNJ-78436735, vaccine effectiveness was found to be greater than 50% after two weeks.
Conclusions
The current study provided real-world evidence on the risk of unvaccinated individuals against SARS-CoV-2. Unvaccinated individuals were four times more likely to contract COVID-19 as compared to vaccinated individuals. Therefore, unvaccinated individuals are advised to get vaccinated, as it will not only protect them but also the community against severe SARS-CoV-2 infections.
Limitations
The current study had two major limitations. First, since the vaccination statuses were self-reported, there were chances of errors and missing data. Second, the data for the study were collected at retail settings, which makes it likely to represent lesser severe infections than those identified in inpatients.
- Tabak, Y. P., Sun, X., & Brennan, T. A., et al. (2021). Incidence and Estimated Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Among Persons Tested in US Retail Locations, May 1 to August 7, 2021. JAMA Network Open. doi:10.1001/jamanetworkopen.2021.43346.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Coronavirus, Coronavirus Disease COVID-19, Epidemiology, Pandemic, Polymerase, Polymerase Chain Reaction, Public Health, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article
