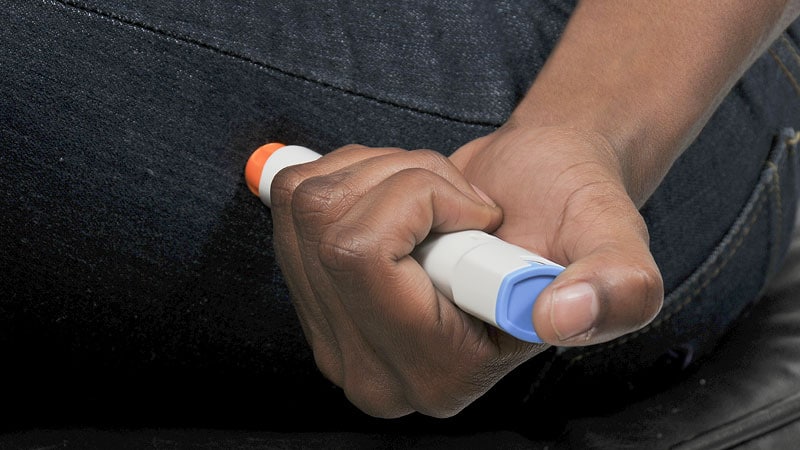
Epinephrine is the only effective treatment for serious allergic reactions called anaphylaxis, yet people with prescriptions for the lifesaving medication often don’t carry their auto-injectors, and many hesitate to use them.
Tonya Winders, CEO and president of the Allergy & Asthma Network, a nonprofit education and advocacy organization, has a teenager with food allergies. “I know the angst it causes her to have that constant reminder that every time she eats, she could be at risk for anaphylaxis,” she says, “not to mention inconvenience, the size of it, the fear of the needle.”
Research shows that many people feel much the same way.
In a survey of more than 1,200 families whose children were prescribed epinephrine auto-injectors for peanut allergies, more than half the parents said they were afraid to use the devices. A review of anaphylaxis cases around the globe found that less than a quarter of children and just 7% of adults with anaphylaxis got epinephrine before going to the hospital – again, underscoring the underuse of this lifesaving medication.
This data is concerning because a delay in getting epinephrine is associated with a higher risk of dying from anaphylaxis.
At the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting, held February 25-28 virtually and in Phoenix, AZ, researchers discussed barriers to epinephrine use and presented new data on needle-free epinephrine products, which could become available next year.
Besides anxiety about needles, other things contribute to not using epinephrine. For some, cost can be an issue. Many families pay $50 to $150 per year for epinephrine auto-injectors to keep one at school, another at a child care place, and one at home. Prices range widely, depending on the product, insurance, and pharmacy. And costs can jump unpredictably.
Carrying the devices can also be a bit of a hassle. For people who have gone years without an anaphylactic reaction or accidental exposure, it becomes easier to justify leaving the epinephrine at home – “to be like, well, I probably don’t have to take it this time. I don’t have to make sure I have it everywhere I go,” says Winders.
It’s common for children to have allergic reactions, sometimes for the first time ever, at school, camp, or other spaces that don’t routinely stock epinephrine. Even when epinephrine auto-injectors are available, few people are trained to use them properly. And even among people who have been prescribed these devices, when tested, 84% failed to show the correct way to use them.
Emergency Room Challenges
Despite recognizing anaphylaxis and even having epinephrine on hand, there’s a less obvious reason some people hesitate to use the medication: reluctance to go to the emergency room.
When teaching families how to manage food allergies, doctors typically tell them to take a child with anaphylaxis to the ER for evaluation after giving epinephrine. This is “the way most allergy action plans are written,” says Andrew Winslow, MD, an allergy/immunology fellow at the University of North Carolina.
Part of the reason is the possibility that some anaphylactic reactions could be biphasic, meaning symptoms crop up again within 72 hours of the first event despite no allergen re-exposure after the first set of symptoms completely go away.
Just how often these biphasic reactions occur isn’t so clear. According to limited published data, Winslow says, the proportion of anaphylaxis cases that are biphasic can range from less than 1% to nearly 15%, though some reports include non-food triggers such as drugs and insect stings.
To better define how often biphasic reactions happen for food-induced anaphylaxis cases, Winslow and colleagues reviewed patient records of children highly allergic to peanuts who had oral food challenges where they ate peanuts as participants in oral immunotherapy and sublingual immunotherapy trials at UNC.
Among 113 oral food challenges, 44 required giving epinephrine – and six of those needed two doses. The researchers then looked to see how many of those cases were considered biphasic.
They found just one: A 7-year-old boy developed a skin rash and gastrointestinal symptoms within an hour of his peanut challenge, then 2 hours later started wheezing and coughing and had another rash.
Bottom line: In a controlled clinical trial setting with careful data collection, “biphasic anaphylaxis is very rare,” says Winslow, who presented these findings on a poster at the AAAAI meeting.
Another question is how long epinephrine-treated patients should be watched in the hospital after anaphylaxis goes away: Two hours? Six hours? Overnight?
To determine the health and economic consequences of prolonged observation in the ER, a simulation led by pediatric allergist Marcus Shaker, MD, of Dartmouth Geisel School of Medicine found that 6 hours of observation gives “a bit more of a marginal negative predictive value but not much,” compared to 1 hour of observation. Yet prolonged observation has a high price tag – $68,000 to $230,000 for each case of biphasic anaphylaxis observed, Shaker reported at the AAAAI meeting.
So the issue becomes how to identify who’s at higher risk for biphasic anaphylaxis. Based on previous studies, biphasic reactions are more likely in people whose first reactions were severe – “like, if you’ve been intubated or ventilated or required more than two doses of epinephrine,” Winslow says.
Using epinephrine “doesn’t necessarily have to be linked to calling 911,” Shaker says. But “if you’re monitoring at home, it’s really important to make sure you have access to help and to emergency services and additional epinephrine if you need it. These decisions are definitely contextual.”
Needle-Free Products
In the meantime, several companies are hoping to ease the anxiety around using epinephrine by coming up with needle-free products.
ARS Pharmaceuticals of San Diego, CA, and Bryn Pharma of Raleigh, NC, are working on nasal spray versions, and Aquestive Therapeutics of Warren, NJ, is creating a sublingual product – akin to the Listerine breath strips that dissolve when placed on the tongue.
“The intranasal epinephrine would be a breakthrough, especially if it is easier to carry, cheaper and, most importantly, effective,” says Brian Schroer, MD, director of allergy and immunology at Akron Children’s Hospital in Akron, OH.
ARS Pharmaceuticals did research comparing how the human body absorbs its nasal spray product (Neffy 1 milligram) to similar data from prior analyses of auto-injectors (EpiPen 0.3 mg and Symjepi 0.3 mg) and manual intramuscular injection. In this analysis, which the company presented on a poster at the AAAAI meeting, epinephrine reached its highest concentration in the blood fastest with EpiPen (20 minutes), followed by Symjepi and Neffy (both 30 minutes) and intramuscular injection 0.3 mg (45 minutes).
The company developed the 1 mg Neffy to be comparable to 0.3 mg intramuscular (IM) injection, which has been the gold standard at hospitals for decades and is considered clinically equivalent to epinephrine auto-injectors. There is no evidence that faster is better within a 10- to 45-minute time period to highest blood concentration, says Richard Lowenthal, co-founder and CEO of ARS Pharmaceuticals.
Bryn Pharma reported that its nasal spray product (BRYN-NDS1C 6.6 mg) reached highest blood concentration in about 20 minutes, regardless of whether self-administered or given by trained professionals.
Aquestive Therapeutics is developing a postage stamp-sized product (AQST-109) that delivers epinephrine and begins dissolving when placed under the tongue. No water or swallowing is required, and its packaging is thinner and smaller than a credit card. Analyses reported at the AAAAI meeting showed that the epinephrine reaches highest concentration in the blood in about 15 minutes.
After the 1-milligram nasal spray analyses presented at the allergy meeting, ARS Pharmaceuticals plans to present newer data with its 2-milligram dose (for people weighing more than 30 kilograms, or about 66 pounds) at the American Academy of Pediatrics conference this fall. The company expects approval and launch of the 2-milligram nasal spray in 2023.
“Having a non-needle delivery device would help many people overcome that fear and hopefully increase use in anaphylaxis,” said David Stukus, MD, an allergist-immunologist and professor of clinical pediatrics at Nationwide Children’s Hospital in Columbus, OH, who was not involved with any of the studies on EpiPen alternatives.
And “it’s not just food allergy” he says – anaphylaxis can occur from venom stings, medications, or unknown causes.
Sources
Annals of Allergy, Asthma, and Immunology: “Factors contributing to underuse of epinephrine autoinjectors in pediatric patients with food allergy.”
Tonya Winders, CEO and president, Allergy & Asthma Network.
Allergy: “A majority of parents of children with peanut allergy fear using the epinephrine auto-injector.”
The Journal of Allergy and Clinical Immunology: In Practice: “Community Use of Epinephrine for the Treatment of Anaphylaxis: A Review and Meta-Analysis.” “Fatal Anaphylaxis: Mortality Rate and Risk Factors.”
Journal of Asthma and Allergy: “Underuse of epinephrine for the treatment of anaphylaxis: missed opportunities.”
AAAAI: “Biphasic Anaphylaxis: Integrating Best Value Care with Patient Safety,” “Moving Away from Routine Emergency Department Evaluation After Treatment of Anaphylaxis – A Retrospective Review of Epinephrine Usage Among High-Risk Peanut-Allergic Children,” “Pharmacokinetics and Pharmacodynamics of Neffy (1 mg) compared to EpiPen (0.3 mg) and Manual IM injection (0.3 mg) – an Integrated Analysis,” “Subanalysis of an Open-Label, Crossover Study to Assess the Relative Bioavailability of Self-administered Nasal Epinephrine Compared to Administration by Trained Health Personnel in Healthy Adult Subjects,” “A Phase 1, Randomized Study Evaluating the Safety, Tolerability, Pharmacokinetics (PK) and Pharmacodynamics (PD) of Single Ascending Doses of Epinephrine Prodrug 109 Sublingual Film (AQST-109) in Healthy Male Volunteers.”
Andrew Winslow, MD, allergy/immunology fellow, University of North Carolina.
Marcus Shaker, MD, pediatric allergist, Dartmouth Geisel School of Medicine.
ARS Pharmaceuticals, San Diego, CA.
Bryn Pharma, Raleigh, North Carolina.
Aquestive Therapeutics, Warren, NJ.
Brian Schroer, MD, director of allergy and immunology, Akron Children’s Hospital, Akron, OH.
Richard Lowenthal, co-founder, CEO, ARS Pharmaceuticals.
David Stukus, MD, professor of clinical pediatrics, Nationwide Children’s Hospital, Columbus, OH.
Source: Read Full Article