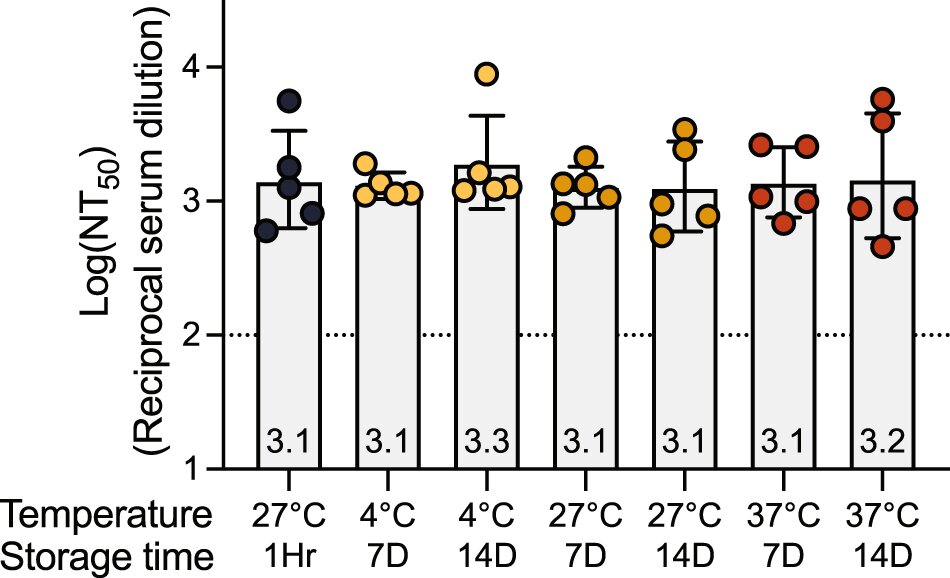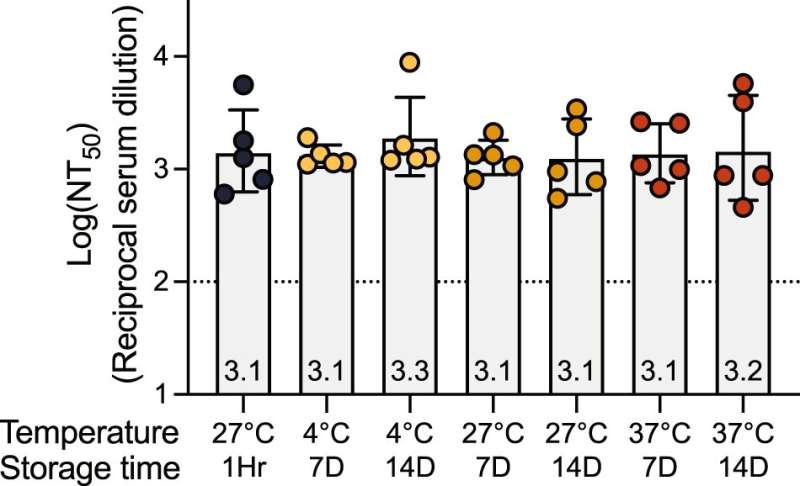
A low-cost, protein-based COVID-19 vaccine tested in rhesus monkeys by Stanford Medicine researchers and colleagues offered immunity against known variants for at least one year. Researchers hope the vaccine, which can remain unrefrigerated for up to two weeks and may be especially beneficial for infants, will help alleviate the need for boosters while improving herd immunity around the world.
If the vaccine succeeds in human trials, it could be an alternative to the mRNA vaccines widely used for COVID-19, without drawbacks such as high expense and low-temperature storage requirements. Protein-based vaccines, which use protein fragments of the target virus rather than the whole virus, have been used for decades to protect against diseases such as shingles and hepatitis.
“Our motivation was to come up with a vaccine that would provide worldwide access to vaccination,” said Peter Kim, Ph.D., the Virginia and D.K. Ludwig Professor in Biochemistry. “In the case of the mRNA vaccines, for example, they are expensive, difficult to make and require storage in freezers. So, we wanted to solve those problems with this vaccine.”
The new vaccine is called Delta-C70-Ferritin-HexaPro with an aluminum adjuvant—the substance that stimulates a stronger immune response. It’s known as DCFHP-alum for short.
Kim is the senior author of a paper, published April 17 in Nature Communications, that describes the vaccine. First co-authors include Payton Weidenbacher, Ph.D., a former graduate student in chemistry; biochemistry research scientist Mrinmoy Sanyal, Ph.D.; and former research scientist in biochemistry Natalia Friedland, Ph.D.
Broad-spectrum protection
A large fraction of the world’s population is either in need of a booster or is still unvaccinated because of the temperature requirements and cost. In some African countries—the continent with the least vaccinations per capita—less than 10% of the population is vaccinated, according to the Africa Centers for Disease Control and Prevention. DCFHP-alum can be distributed at low cost because it can be produced in large quantities and is stable without refrigeration. Its adjuvant is aluminum hydroxide, or alum, which is inexpensive and widely used because of its safety rating, including in infants.
Kim anticipates that the vaccine would work well as the initial version infants receive. An infant’s immune response could be more protective, Kim said, if the infant is first vaccinated with a broad-spectrum vaccine that targets many strains like DCFHP-alum does.
The vaccine provided immunity in rhesus monkeys against SARS-CoV-1, a distant relative of the SARS-CoV-2 lineage. Unlike the bivalent Pfizer/BioNTech and Moderna mRNA vaccines, which contain the original Wuhan strain and the latest mutated strain, DCFHP-alum was developed using only the first strain of the spike protein.
Such an approach dodges “variant chasing,” in which researchers keep changing the booster recipe to keep pace with virus mutations.
“What’s exciting is that we could increase herd immunity if these results translate to humans because people are tired of getting vaccines every three or four months,” Kim said.
The recipe
In developing the DCFHP-alum vaccine, scientists made several changes to the spikes found on the surface of the coronavirus.
They first stabilized the spikes: In hijacking our cells to create new virus particles, the coronavirus grabs proteins on our cells using the spikes on its surface. Once the proteins attach to the spikes, the spikes turn inside-out to fuse with our cells. By modifying the spikes to make them more rigid so that they’re not able to turn inside-out, vaccines can prevent this tampering.
For the second change, they fused ferritin, a nanoparticle that offers up antigens to the immune system, to the spikes. Using nanoparticles in addition to stabilizing the spike proteins is a better stimulant of the immune system, Kim said, partly because the nanoparticles are captured by dendritic cells. These cells catch foreign antigens and transport them to lymph nodes where they encounter B cells and T cells—essential components of an immune reaction.
The third change was that the researchers deleted the last 70 amino acids, the building blocks of proteins, of the spike closest to the membrane of the virus. Kim described the region as “a distraction for the immune system.” People make a strong antibody response against this region, but the antibodies produced do not neutralize the virus. By removing that part of the protein, the scientists’ hypothesis was that the vaccine would stimulate the immune system to make more antibodies that neutralize the virus.
Once the vaccine was developed, the team immunized 10 male rhesus monkeys, then divided them into two groups: one that received a booster 21 days later and the other 92 days later. The animals were then tested for antibodies that confer immunity to the virus. Both showed resistance after immunization, and it lasted at least 250 days. The booster given after 92 days elicited a more robust immune response than the one given after 21 days.
The researchers gave the monkeys a second booster on day 381, which nearly eliminated the immune response differences seen in the two groups. Both had a significant immune response after the second boost, demonstrating immune system memory.
Kim hopes to see similarly promising results in people. Clinical trials will start within the next few months.
“With this promising vaccine, if it passes clinical trials, we can target a large fraction of the world’s unvaccinated population or those in need of a booster,” Kim said.
More information:
Payton A.-B. Weidenbacher et al, A ferritin-based COVID-19 nanoparticle vaccine that elicits robust, durable, broad-spectrum neutralizing antisera in non-human primates, Nature Communications (2023). DOI: 10.1038/s41467-023-37417-9
Journal information:
Nature Communications
Source: Read Full Article