In research on the eye disease AMD, the experimental animals used are often young male mice. This is not optimal for the development of new treatments, as the disease most often affects the elderly—and women.
The eye disease Age-related Macular Degeneration (AMD) is the most frequent cause of vision loss and blindness among elderly people in the Western world.
The most aggressive form is so-called “wet AMD”—a disease in which the formation of new blood vessels in the underlying choroid of the retina results in fluid and swelling in the eye.
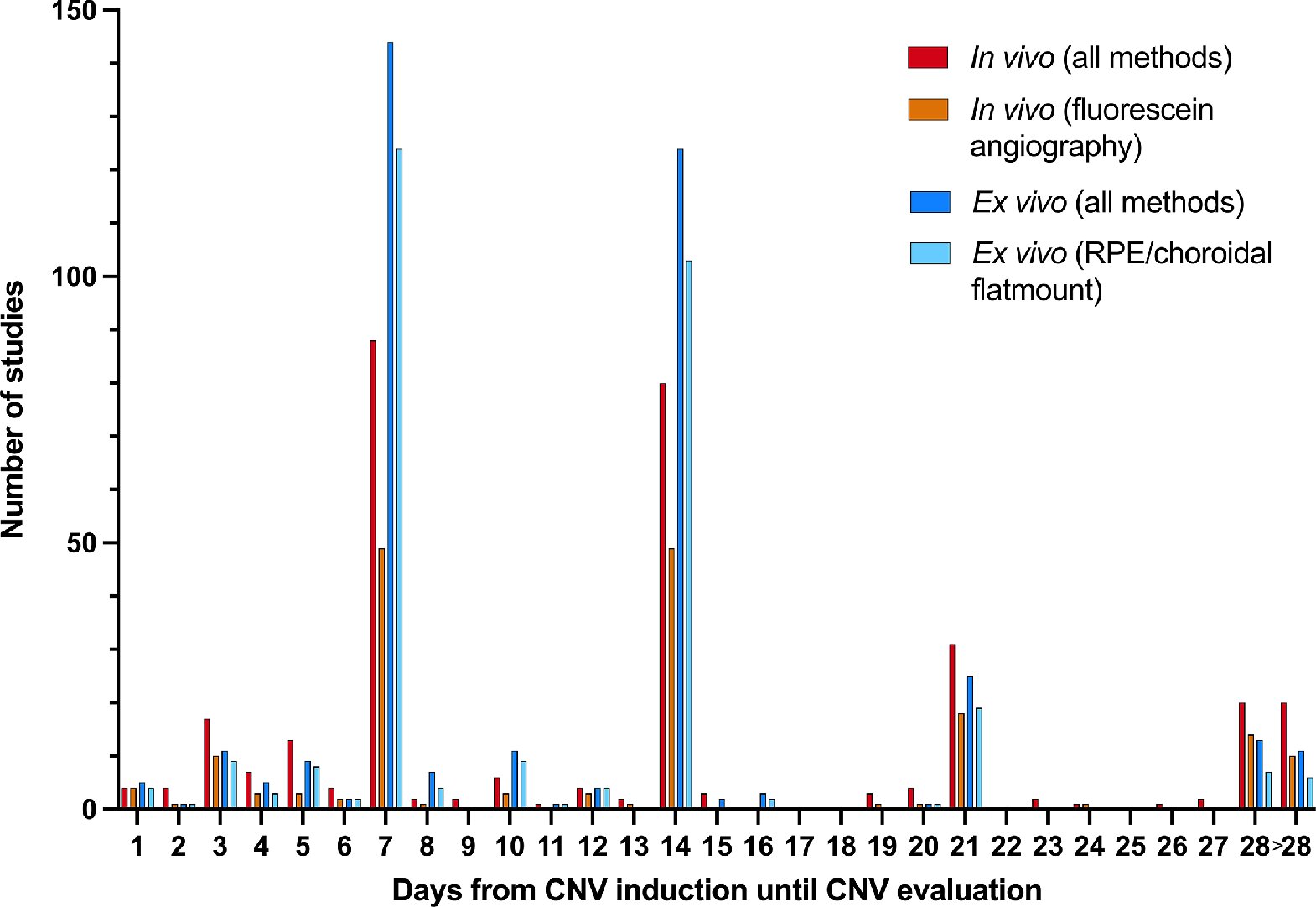
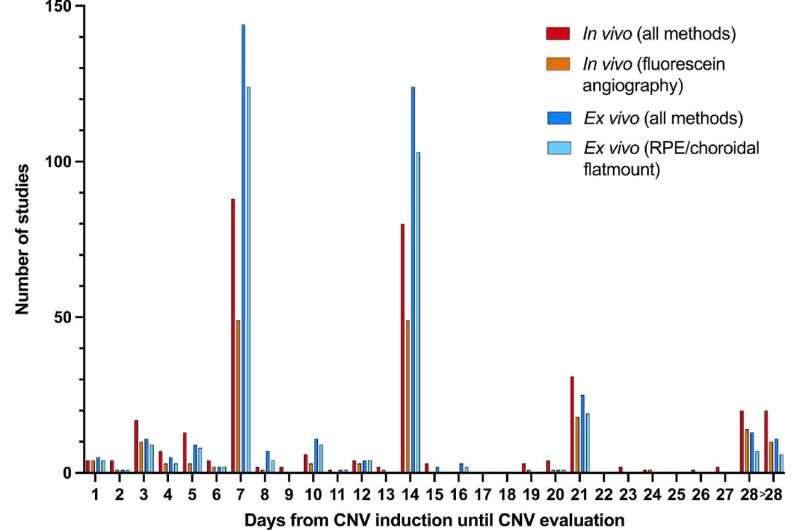
In a new study from Aarhus University which has just been published in Investigative Ophthalmology & Visual Science (IOVS), the authors have examined the past five years of studies in a model for the disease corresponding to 380 scientific articles. Research into the mechanisms behind the disease—and thus the development of new treatment modalities—is often based on laboratory animals, in which new blood vessels are artificially created in the retina, typically using lasers.
The review of the many studies shows an imbalance in the use of laboratory animals; researchers usually choose to use young, healthy male mice, even though they know that both the sex and the age of the mice play a role in the degree of vessel formation.
“It is predominantly young, healthy, male mice that are used for research on this type of vessel formation. This makes sense from an ethical, financial and time perspective, as mice are inexpensive compared with larger laboratory animals, and the studies can be carried out relatively quickly. The biological variation can also be limited. However, AMD is a disease that most frequently occurs in the elderly population, and the disease occurs just as frequently in women—perhaps even slightly more frequently, when you look at ‘wet AMD’,” says medical doctor and Ph.D. student Bjørn K. Fabian-Jessing, who is first author on the study.
Previous studies have shown a discrepancy between the treatment effects seen in animal studies and in clinical trials in humans.
“We are apparently seeing a ‘translational gap’, because research does not take place in the most relevant animal model. Greater biological variation could be achieved by, for example, using more female mice and/or older animals. This would probably boost the possibility of translating the research to clinical trials—and perhaps, ultimately, reduce the number of laboratory animals, as the results from the preclinical animal testing would in that case be easier to extrapolate,” says Bjørn K. Fabian-Jessing.
Poor reporting
In addition to describing the animal models, the article also focuses on the degree of reporting.
“We can see that many studies fail to report important variables, such as how many mice have been used. This makes it difficult to compare studies and assess the value of the results. This is worrying, because it makes it impossible to reproduce the results, and this diminishes their reliability,” says Bjørn K. Fabian-Jessing.
Call for better guidelines
Data in the area of the eye has not previously been collected and synthesized in this way.
“The new study calls for the development and updating of the guidelines for these animal models,” says Bjørn K. Fabian-Jessing. He is backed up by Professor Thomas Corydon, who is also behind the study:
“The implementation of concrete guidelines will improve the quality, value and comparability of animal testing, which will naturally be of great benefit to the research, understanding and future treatment of eye diseases,” says Thomas Corydon.
He encourages leading researchers in the area to draw up consensus-based guidelines, as has previously been done for preclinical research in other medical specialties.
Source: Read Full Article