SARS-CoV-2 causes the pandemic coronavirus disease COVID-19, that is more harmful for elderly people, who show more severe symptoms and are at higher risk of hospitalization and death. A group of Italian and American researchers led by Fabrizio d’Adda di Fagagna now reports that the expression of the cell receptor for the virus, ACE2, which is essential for mediating cell entry of the virus, increases in the lungs of aging mice and humans. They further show that ACE2 expression increases upon telomere shortening or dysfunction—common hallmarks of aging—in cultured human cells and in mice. This increase depends on a DNA damage response elicited by dysfunctional telomeres. The findings published today by EMBO Reports provide one possible molecular explanation for the increased sensitivity of elderly people to SARS-CoV-2.
The reasons for the higher probability of severe symptoms and death in the elderly in response to a SARS-CoV-2 infection remain unclear. ACE2 expression has been positively related to patients’ age, for example, in the nasal epithelium, the first point of contact with SARS-CoV-2. Lower ACE2 expression in children relative to adults may explain why COVID-19 is less prevalent in children, and the expression and distribution of the ACE2 receptor may be relevant for the progression and prognosis of COVID-19. The research findings now show that ACE2 protein expression is elevated in aging human and mouse lungs, including in alveolar epithelial type II cells (ATII). In the lungs, ACE2 is mostly found on the surface of ATII cells, and these cells are thus likely the primary target of SARS-CoV-2 infection in the lungs. SARS-CoV-2 mainly spreads via respiratory droplets and the lung is the first target organ of the virus. Indeed, pneumonia is the most common complication seen in COVID-19 patients, at an occurrence of 91%.
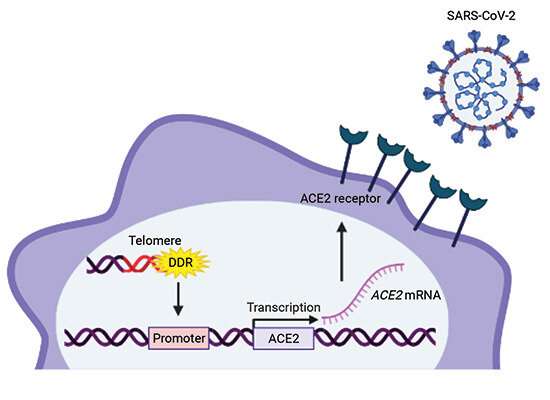
In order to reveal the molecular mechanism underlying the upregulation of ACE2 during aging, the researchers turned to in vitro and in vivo models that recapitulate some key aspects of aging. Aging is associated with telomere shortening and damage in a range of tissues in different species, including humans. Telomeres are the regions at the ends of linear chromosomes that are essential to protect chromosome ends from shortening during repeated cell replication cycles, which would result in the loss of crucial genetic information. When telomeres become critically short, they are sensed as DNA breaks and activate DNA damage response pathways.
D’Adda di Fagagna working at IFOM in Milan and CNR-IGM in Pavia and colleagues either inhibited the general DNA damage response by targeting ATM, a major enzyme of the DNA damage response pathway, or they inhibited the telomeric DNA damage response specifically using telomeric antisense oligonucleotides (tASO). Both approaches prevent ACE2 gene and protein upregulation following telomere damage in aging cultured cells and in mice. The group also used a cell culture model in which the DNA damage response is activated specifically at telomeres in the absence of telomere shortening, with the same results. These findings indicate that it is the DNA damage response activation, rather than telomeric shortening per se, that is responsible for ACE2 upregulation. Understanding the mechanism of age susceptibility to SARS-CoV-2 infection is important for targeted therapeutic approaches, which might in principle include the use of tASO-mediated inhibition of the telomeric DNA damage response.
ACE2 also has a role in the regulation of blood pressure and the balance of fluids and salts and is expressed in other human tissues, for example the heart and kidney. The findings reported here may thus also have broader medical implications beyond COVID-19.
Source: Read Full Article
