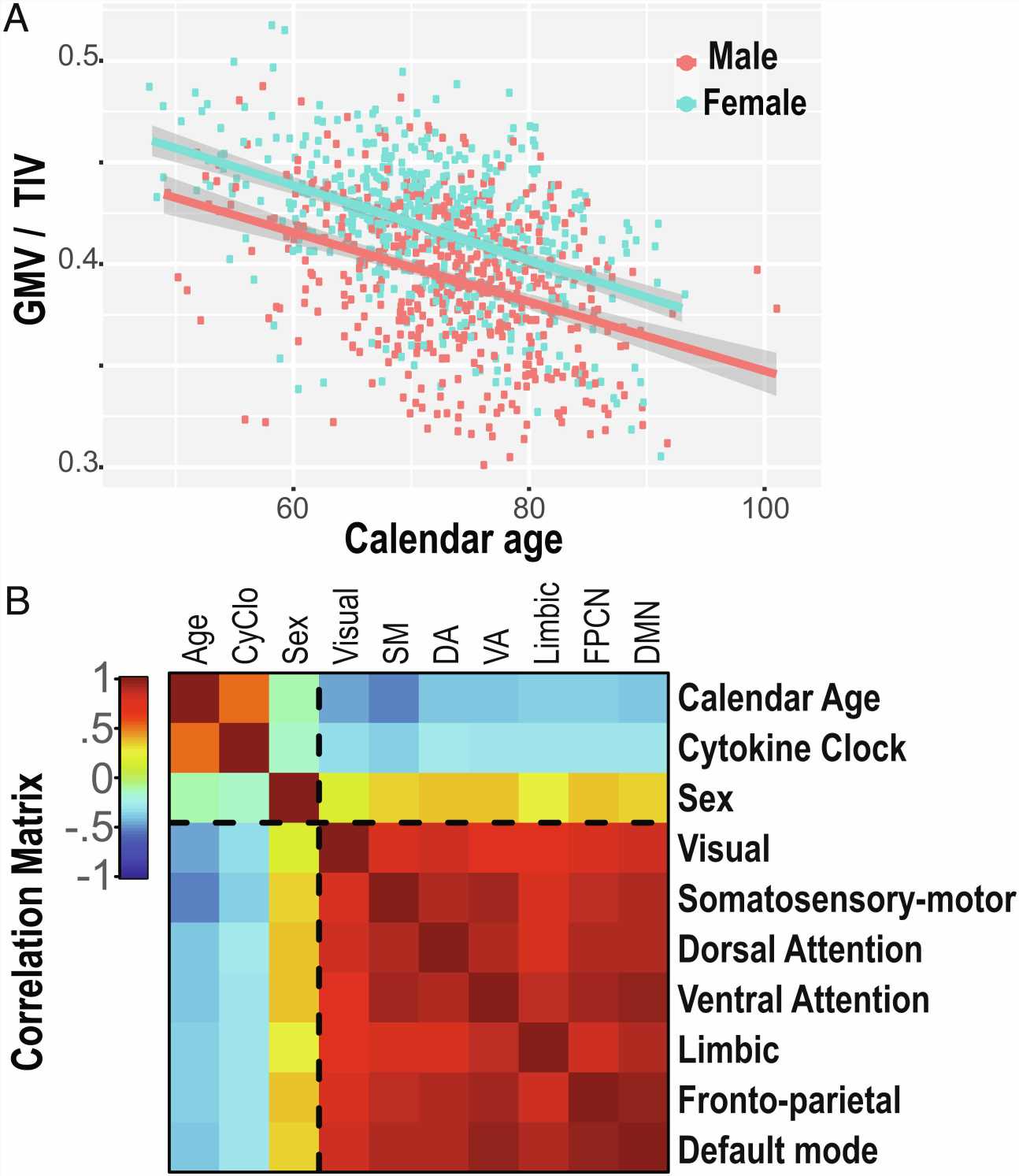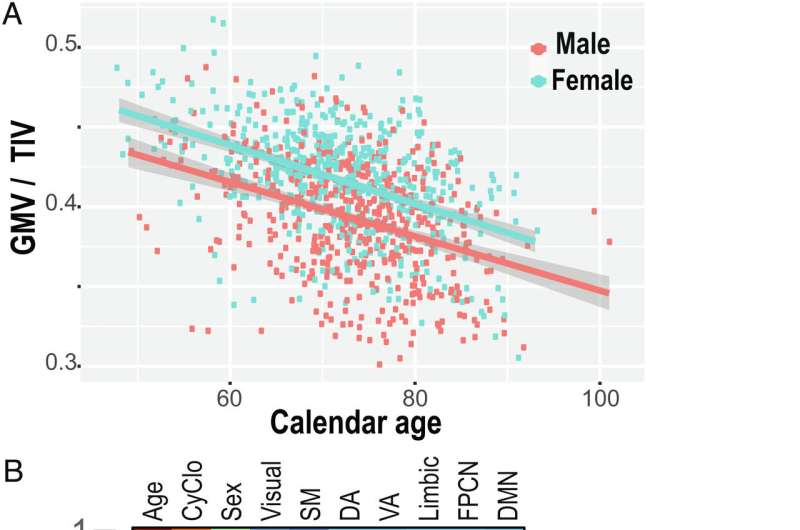
Humans lose brain volume as they age, at an estimated rate of 5% per decade after age 40. But that estimate obscures the role that individual physiology plays in regards to functional brain aging.
Utilizing sophisticated analytic tools, researchers at the Buck Institute and the University of California, San Francisco analyzed 554 patients using volumetric MRIs and blood immune samples taken over the course of a decade.
The results of the study, appearing in Proceedings of the National Academy of Sciences, show that functional brain networks are affected in different ways by aging, gender and blood immune factors.
Researchers, led by bioinformatics scientist Nikola Markov, Ph.D., first looked at circulating blood proteins, identifying patterns of concentrations of inflammatory proteins that increase with age, a process called “inflamm-aging.” The correlation with aging was so clear that the scientists were able to predict the chronological age of a person with striking precision just by analyzing the concentration profile of these proteins. This measure is called CyClo or a “cytokine clock.”
Researchers found that both the cytokine clock and brain shrinkage follow different trajectories depending on gender, with females having a faster ticking cytokine clock even though they were more protected from brain shrinkage overall.
“This work breaks open the black box between aging and neurodegeneration,” says senior author and Buck Institute associate professor David Furman, Ph.D. “We are now able to differentiate between the brain shrinkage caused by aging versus the shrinkage caused by chronic inflammation.
“Basically, if we remove the causality of time and someone has high inflammation they are on track to have a smaller brain in certain areas. In addition, by identifying inflammatory biomarkers of brain aging we have potential targets for both early diagnostics and the prevention of age-related neurodegeneration.”
The findings are based on a canonical correlation analysis. This technique allows researchers to take two sets of variables collected in the same individual and decouple the effects of these variables on a particular outcome, in this case, brain regions that are functionally connected. It also allows scientists to see in the same individual how the parameters correlate to one another.
Researchers in this study analyzed three parameters—age, gender and CyClo—as well as the measures of the volume of seven functional brain networks in the same dataset. Markov says the correlation functions revealed interesting observations.
The variability of chronological age measured in the population constructed the strongest correlation function with the volume of the brain networks dedicated to motor control and body sensing. The gender of the subject correlated with the visual system, ventral attention and frontoparietal networks. The biological age represented by the cytokine clock, CyClo, correlated the most with the limbic, default mode and dorsal attention network which is concerned with orienting one’s focus on a particular task.
“The significance is not just that we’ve mapped the cytokine clock to gray matter, but that we’ve done it by looking at functional networks, areas in which neurons fire together, work together, connect, and interact together,” said Markov. “We also found that although the networks are distributed across the brain, they share common vulnerabilities to the process of getting old.”
Markov says the next challenge is to understand how these immune circulating proteins directly impact the aging brain and why there are some parts of the brain that are more sensitive to peripheral inflammation than others. He says the most impactful immune proteins, or cytokines/chemokines, that showed up in blood samples include IL-6 and TNF alpha which are associated with the chronic inflammation that increases with age, as well as VEGF and PLGF, which seem to be critical for the health of the vascular system, especially the small capillaries.
Researchers also identified MCP-1, Vcam-1 and Eotaxin-1 which are chemokines that change with age and attract immune cells from the blood to cross the brain blood barrier. Markov says when those chemokines cross the brain blood barrier, they cause activation of microglia, a potential initiator of the neurodegenerative process.
Going forward Furman sees two potential uses for the science: “Once we have identified that the most affected networks are also mapping to cognitive impairment in individuals who are diagnosed with Alzheimer’s or Mild Cognitive Impairment, then we have a diagnostic test. The next opportunity involves the question of whether we can modify this cocktail of cytokines to help individuals be more resilient and avoid decline in these different areas of the brain where we saw a decline in function.”
More information:
Nikola T. Markov et al, Age-related brain atrophy is not a homogenous process: Different functional brain networks associate differentially with aging and blood factors, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2207181119
Journal information:
Proceedings of the National Academy of Sciences
Source: Read Full Article