In a recent study published on the bioRxiv* preprint server, researchers assess the impact of extending the interval between primary and booster coronavirus disease 2019 (COVID-19) vaccine doses.

Study: A long interval between priming and boosting SARS-CoV-2 mRNA vaccine doses enhances B cell responses with limited impact on T cell immunity. Image Credit: Numstocker / Shutterstock.com
Background
The standard regimen for administering severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger ribonucleic acid (mRNA) vaccines includes a 21-day and 28-day interval for Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 vaccines between two doses, respectively.
Given the emerging issue of vaccine scarcity and the considerable extent of protection elicited in non-high-risk individuals by the first vaccine dose, several public health agencies have implemented vaccine regimens with longer intervals between doses.
About the study
In the present study, researchers compared the B- and T-cell responses elicited by two COVID-19 mRNA vaccine doses when administered at an interval of three to four weeks as compared to a 16-week interval in SARS-CoV-2 naive persons.
The immune responses exhibited by two independent groups of healthcare workers (HCWs) vaccinated with two doses of COVID-19 mRNA vaccines were assessed. The two cohorts had different time intervals between prime and booster vaccinations. These included the long interval (LI) with a 16-week interval and the short interval (SI) cohort that had a three- to four-week interval between vaccine doses.
Patient blood samples were collected at four time points, including baseline (B) before vaccination, three weeks post-first vaccination (D1), one to three weeks after the second vaccination (D2), and 10 to 16 weeks after the second vaccination (M2).
SARS-CoV-2-specific B-cells were evaluated by focusing on the SARS-CoV-2 spike (S) receptor-binding domain (RBD) to reduce the inclusion of B-cells that were cross-reactive with endemic coronaviruses. The magnitude of RBD-specific B-cells was also explored in the two cohorts.
The researchers assessed whether the interval between two vaccine doses qualitatively affected the development of antigen-specific B-cells by estimating immunoglobulin A (IgA), IgD, IgG, and IgM expression correlated to the RBD-specific B-cells. Differentiation of RBD-specific B-cells was also assessed by quantifying CD27 and IgD co-expression.
Additionally, SARS-CoV-2 S-specific T-cell responses were estimated at four different time points across the two cohorts. A T-cell receptor (TCR)-dependent activation induced marker (AIM) assay was used to identify antigen-specific T-cells.
Intracellular cytokine staining (ICS) was used for functional profiling. The ICS assay also measured effector molecules, including interferon-ɣ (IFN-ɣ), interleukin-2 (IL-2), tumor necrosis factor α (TNF-α), IL-10, IL-17A, and CD107a.
Study findings
Most of the participants did not have any signal corresponding to RBD-specific B-cells at baseline; however, B-cell responses were evident after priming with a single COVID-19 mRNA vaccine dose. These responses were similar for both SI and LI groups at the D1 time point.
Conversely, the second vaccine dose induced robust recall responses in the LI cohort at D2, while a plateau was noted in the SI group that led to significant statistical variations between SI and LI groups at D2.
Antigen-specific B-cell responses decreased at M2 across both cohorts, with a comparatively lower decline observed in SI as compared to LI cohorts. SI participants had no temporal association between post-prime and post-boost RBD+ B-cell responses, whereas a robust positive association was observed in the LI group. Similarly, RBD+ B-cell responses were correlated with stronger memory responses in LI cohorts.
RBD-specific B-cells were primarily IgG+ among LI participants at M2 and D2 time-points, whereas significantly lower IgG+ levels were observed in SI cohort members. Unswitched IgD+ and IgM+ RBD-specific B-cells were also reported after booster vaccination in the SI cohort at the M2 and D2 time points, with a higher proportion of IgM RBD+ B-cell responses identified in LI participants at D2.
For both study cohorts, the M2 and D2 time-points highlighted the dominance of an atypical double-negative (DN) CD27– and IgD–. Class-switched memory IgD CD27+ RBD-specific B-cells were also observed in the LI group at D2, which was reduced at M2.
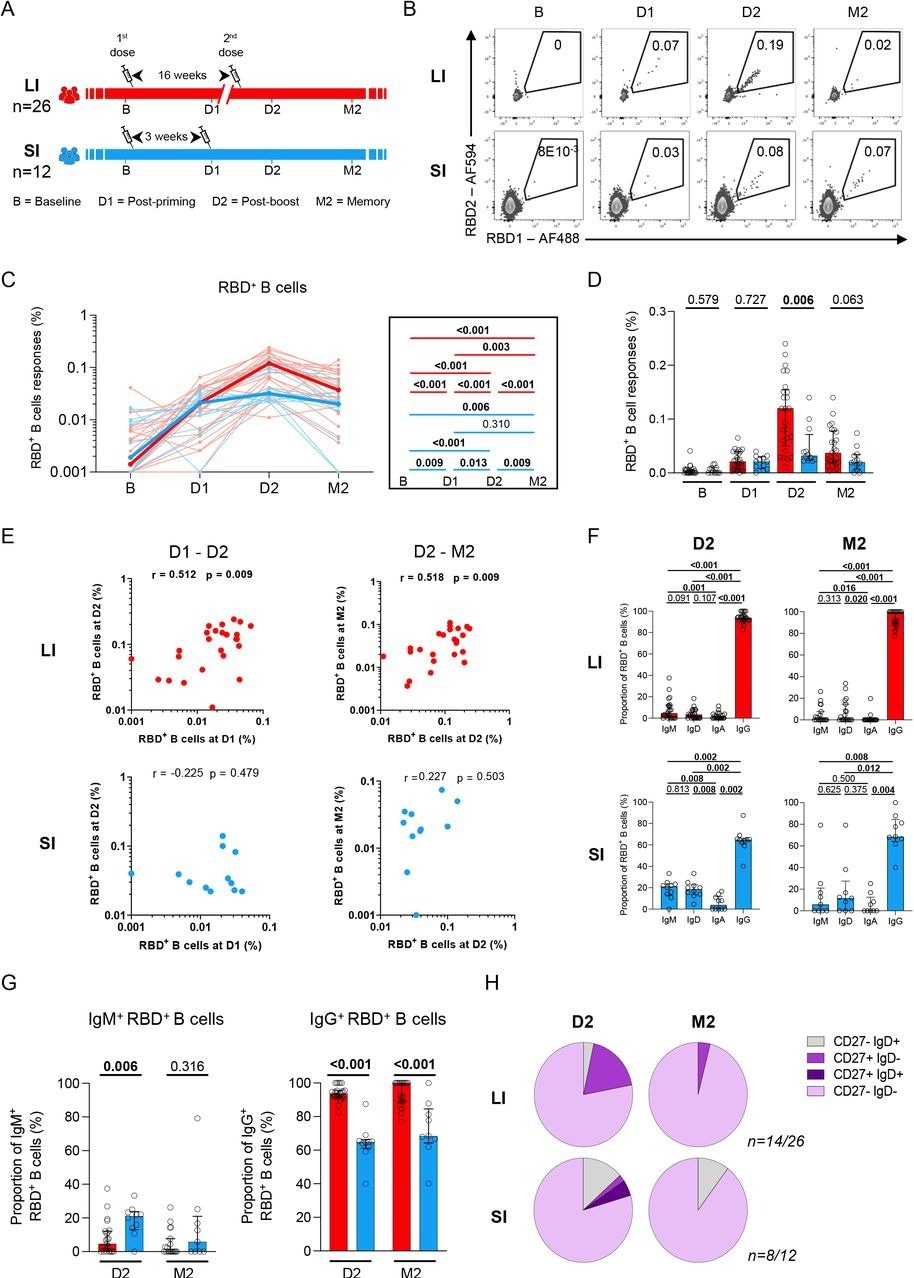
A 16-week delayed boost enhances the magnitude and maturation of B cell responses. (A) Schematic representation of study design. Blood samples were analyzed at 4 time points in the long (red) interval (LI) and short (blue) interval (SI) cohorts: baseline (B); 3 weeks after priming (D1), 1-3 weeks after boost (D2) and 10-16 weeks after boost (M2). (B) Representative examples of RBD-specific B cell responses. (CD) Kinetics of RBD-specific B cell responses in LI (red) vs SI (blue) cohorts. (C) The bold line represents cohort’s median value. Right panel: Wilcoxon tests. (D) Inter-cohort comparisons. Bars represent medians ± interquartile ranges. Mann-Whitney tests are shown. (E) Scatter plots showing temporal RBD+ B cell correlations in the LI and SI cohorts. r: correlation coefficient. Significant correlations by Spearman tests (p<0.05) are shown in bold. (F) Frequencies of IgD, IgM, IgA and IgG-positive cells within RBD-specific B cells within each cohort, paired comparisons with Wilcoxon tests. (G) Proportion of IgM+ and IgG+ cells among RBD+ B cell cells, with Mann-Whitney tests for comparison between the LI and SI cohorts. (H) Proportion of IgD+/-and CD27+/- populations in RBD-specific B cells. In H, only D2 and M2 provided enough events for analysis. In C-E n=26 for long-interval (LI), n=12 short-interval (SI). In F-H n=14 for long-interval (LI), n=8 short-interval (SI).
Notably, rare naive-like IgD– CD27+ were found in the LI cohort, while a higher number of IgD+ CD27– RBD-specific B-cells were identified in the SI cohort. This indicated that, in comparison to the standard SI regimen, more robust RBD+ B-cell responses were elicited when the second vaccine dose was administered 16 weeks after the first dose. A three-week interval also resulted in lower intensities of RBD+ B-cell responses that were related to a weaker association of early post-boost responses with memory responses.
Initial variations in the magnitudes associated with S-specific CD4+ and CD8+ T-cell responses were observed between the cohorts at baseline and after the prime vaccine dose. By D2 and M2, no significant differences in CD4+ T-cell responses were observed.
Conclusions
Overall, the current study demonstrates that a 16-week interval between two consecutive mRNA COVID-19 vaccine doses enhanced the magnitude and maturation of SARS-CoV-2 RBD-specific B-cell responses. Although there may be a benefit to extending the interval between COVID-19 vaccine doses, further research is needed to determine whether this protection persists against new SARS-CoV-2 variants.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Nicholas, A., Sannier, G., Dube, M., et al. (2022). A long interval between priming and boosting SARS-CoV-2 mRNA vaccine doses enhances B cell responses with limited impact on T cell immunity. bioRxiv. doi:10.1101/2022.08.03.502672. https://www.biorxiv.org/content/10.1101/2022.08.03.502672v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antigen, Assay, B Cell, Blood, CD4, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Healthcare, immunity, Immunoglobulin, Interferon, Interleukin, Interleukin-2, Intracellular, Necrosis, Public Health, Receptor, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Tumor, Tumor Necrosis Factor, Vaccine

Written by
Bhavana Kunkalikar
Bhavana Kunkalikar is a medical writer based in Goa, India. Her academic background is in Pharmaceutical sciences and she holds a Bachelor's degree in Pharmacy. Her educational background allowed her to foster an interest in anatomical and physiological sciences. Her college project work based on ‘The manifestations and causes of sickle cell anemia’ formed the stepping stone to a life-long fascination with human pathophysiology.
Source: Read Full Article
