Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a positive-sense single-stranded RNA virus that is contagious in humans. It is the causal agent of the ongoing coronavirus 2019 (COVID-19) pandemic. This virus causes a mild to severe infection and has claimed over 2.95 million lives worldwide. SARS-CoV-2 is the successor to SARS-CoV-1, the virus that caused the 2002–2004 SARS outbreak.
Both SARS and SARS-CoV-2 are characterized by pulmonary edema, hypoxia, fibrosis, activation of the coagulation cascade, and hypercapnia. However, severe SARS-CoV-2 is characterized by lymphopenia and neutrophilia. In severe cases, an increase in the neutrophil-to-lymphocyte ratio (NLR) occurs.
For protecting host cells from harmful invading pathogens, neutrophils release protease, neutrophil extracellular traps (NETs), and reactive oxygen species. However, the over-activation of neutrophils results in various harmful effects on the host cells. When neutrophils are abundantly present, they transmigrate into the lungs' alveolar luminal space and, thereby, cause damage to the lung.
The severity of COVID-19 disease depends on the extent of the damage in the lungs and other organs, which in turn is dependent on the comorbidities of individual patients.
SARS-CoV-2 infects the host cells using its surface Spike (S) protein. The S protein establishes contact with the epithelial cells of the hosts' airway via angiotensin-converting enzyme-2 (ACE2). ACE2 is an enzyme that is highly expressed in the endothelial cells and the polarized alveolar epithelial cells of the lungs. It cleaves the peptide hormone angiotensin-I into Ang (1-9) and Ang (1-7).
The primary function of these peptides is to protect the epithelial and endothelial cells against hyperpermeability and excessive inflammation. For COVID-19 infection, ACE2 is the receptor for SARS-CoV-2.
Researchers have found that the genetic deletion of ACE2 elevates lung inflammation. However, administration of Ang (1-7) significantly improves the condition.
Coronaviruses stimulate the activation of a disintegrin and metalloprotease 17 (ADAM17) and shed the extracellular domain of ACE-2 from the cell's surface, thereby, aggravating inflammatory responses. ADAM17 is also responsible for the production of the active form of the pro-inflammatory cytokine tumor necrosis factor-α 71 (TNF-α). This active form is vital for pro-inflammatory cytokine secretion, which causes cytokine storms.
The cytokine storm elicits excessive neutrophil recruitment, a characteristic feature of severe Covid-19 infection. Even though it is well documented that ADAM17 is activated during SARS-CoV infection, which cleaves ACE-2, there is a gap in the research related to its role in severe SARS-CoV-2.
Although several novel vaccines have been developed that are effective against SARS-CoV-2, there is still an urgent need for pharmacological therapies. Critically ill patients, who cannot be vaccinated, are highly dependent on drugs that can inhibit viral replication and inflammatory responses.
Now, a new study posted to the bioRxiv* preprint server focuses on the pharmacological inhibition of ADAM17, which would stop the excessive production of cytokine and uncontrolled neutrophil recruitment. This would protect an individual from acute lung injury.
The current research has revealed that intranasal or intraperitoneal administration of the ADAM17 inhibitors such as TMI-1 and apratastat can significantly reduce pro-inflammatory cytokine production and recruitment of leukocytes to the lungs. These inhibitors were found to bring about an improvement of the lung's histology. Hence, this research provides a potential strategy to prevent severe COVID-19 infections, which could, in turn, lessen the need for critical care as well as reduce the fatality rate.
Two of the main challenges researchers face are the need for high biosafety level infrastructure and the non-usability of mice, the commonly used animal in pre-clinical studies, as mice are not very susceptible to SARS-CoV-2 infection.
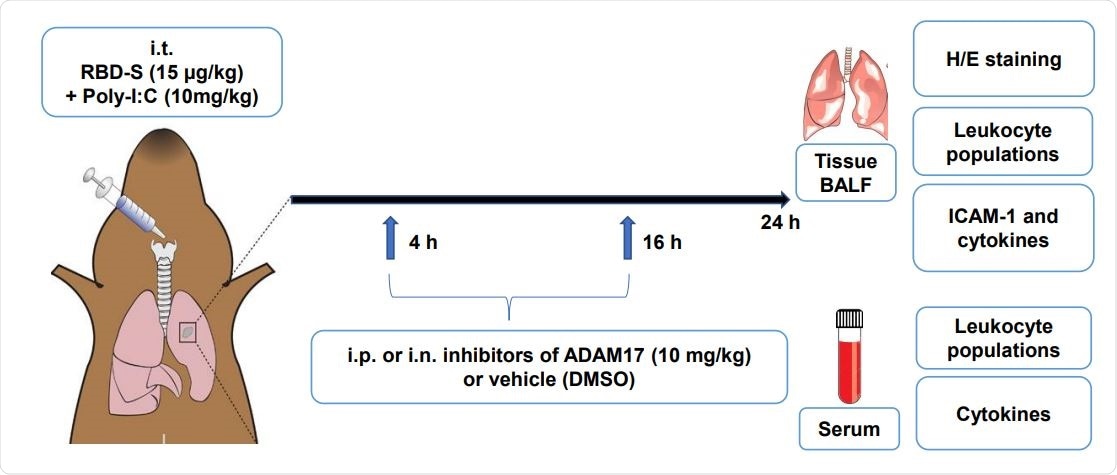
In the present study, researchers have developed a mouse model to analyze the molecular mechanisms associated with COVID-19 without using the actual virus. Their mouse model is based on the TLR3 ligand Poly I: C and the recombinant Receptor Binding Domain (RBD) of the SARS-CoV-2's S protein in C57Bl/6 mice.
Scientists have reported that these mice developed characteristic features that are similar to the ones associated with COVID-19 lung injury, such as edema, increased NLR, fibrosis, and leukocyte infiltration. Thereby, scientists revealed that this simple mouse model could play an important role in studying the molecular mechanisms of COVID-19 pathogenesis.
In the study, only young mice aged 8-10 weeks were considered. However, scientists believe it would be interesting to analyze if older or obese mice could develop severe disease. This is important because such a result would make the model appropriate to study molecular differences in a high-risk population.
To summarize, the study under consideration has shown that apratastat (TMI-005) and TMI-1 could be effectively used to inhibit ADAM17. ADAM17 reduces both the excessive recruitment of neutrophils to the lungs and the cytokine storm. Additionally, it also improves NLR. Apratastat (TMI-005) and TMI-1 are two structurally similar, orally bioavailable, and highly specific inhibitors of ADAM17, which belong to the thiomorpholine sulfonamide hydroxymate family. These compounds also inhibit matrix-metalloproteases (MMP). Thereby, researchers recommended these drugs for clinical trials to investigate their efficacy in severe COVID-19 patients.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Lartey, L.N. et al. ADAM17 inhibition prevents neutrophilia and lung injury in a mouse model of Covid-19, bioRxiv 2021.04.10.439288, doi: https://doi.org/10.1101/2021.04.10.439288, https://www.biorxiv.org/content/10.1101/2021.04.10.439288v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Cell, Coronavirus, Coronavirus Disease COVID-19, Critical Care, Cytokine, Drugs, Edema, Efficacy, Enzyme, Fibrosis, Genetic, Histology, Hormone, Hypoxia, Inflammation, Leukocyte, Ligand, Lungs, Lymphocyte, Lymphopenia, Mouse Model, Necrosis, Neutrophils, Oxygen, Pandemic, Peptide Hormone, Peptides, Preclinical, Protein, Pulmonary Edema, Receptor, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Tumor, Virus

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article
