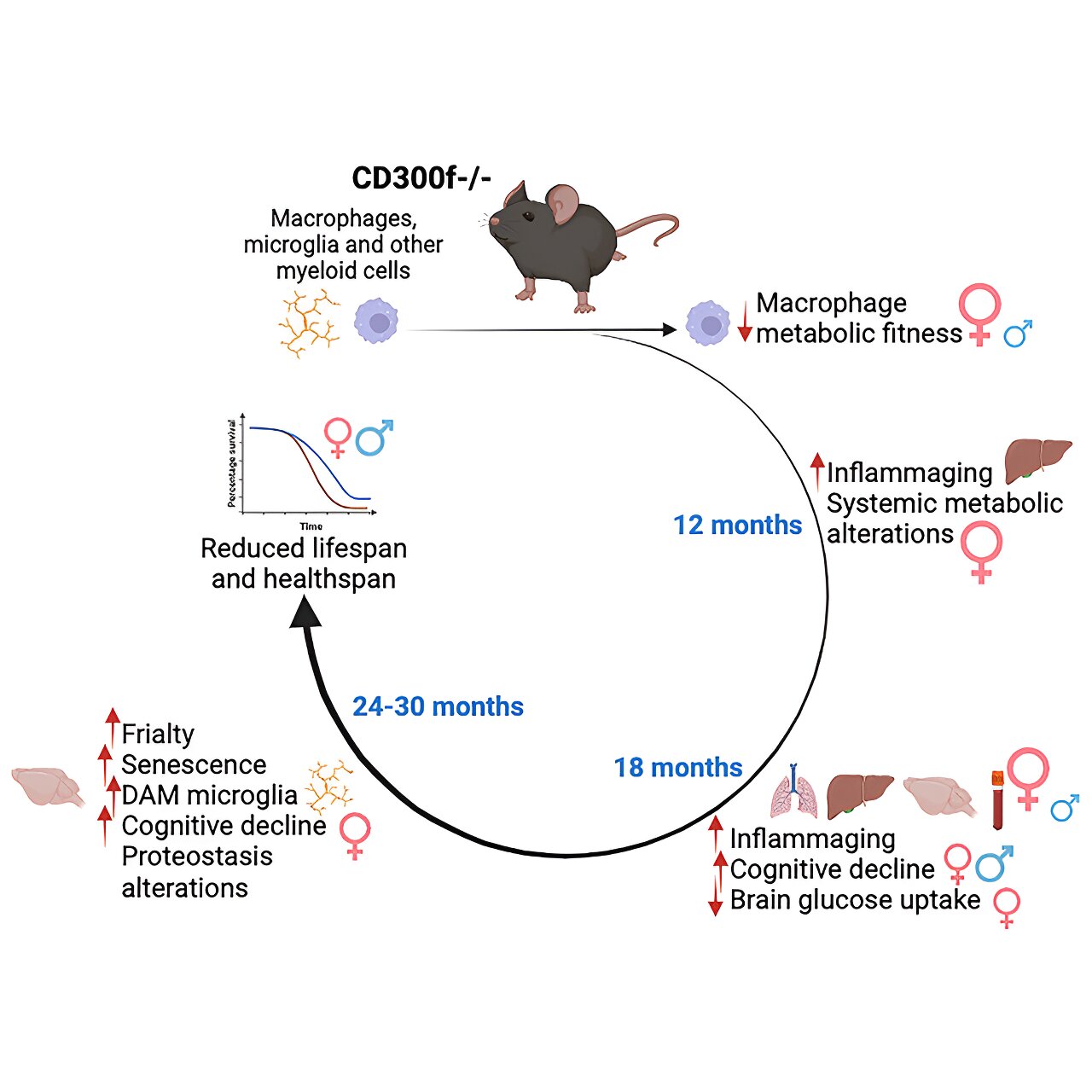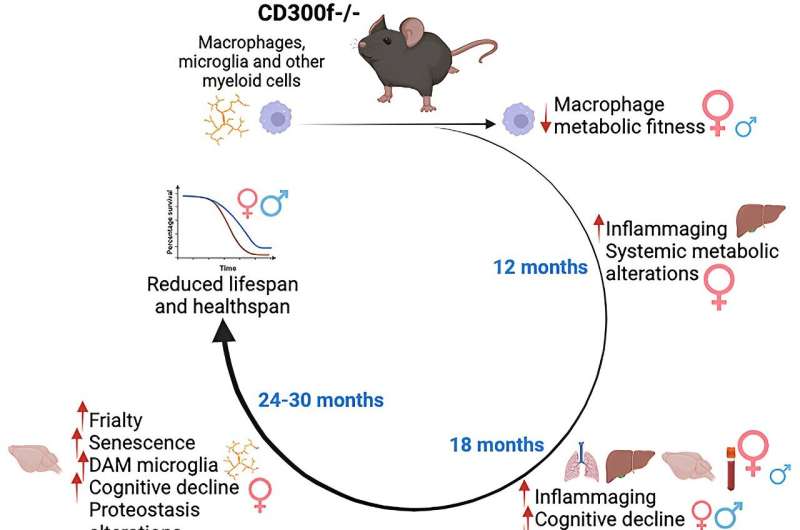
Life expectancy and healthy aging in mice can be determined by a protein present in some cells of the immune system, according to a study published in the journal Cell Reports. When this protein—known as the CD300f immune receptor—is absent, animal models have a shorter life expectancy and suffer from pathologies associated with cognitive decline and premature aging, especially in females.
“Our study indicates that alterations in immune system cells, for instance, in macrophages and microglia, can determine the healthy aging degree in mice,” notes Hugo Peluffo, leader of this study and member of the Faculty of Medicine and Health Sciences and the Institute of Neurosciences (UBneuro) of the University of Barcelona.
Understanding how the CD300f immune receptor—and the myeloid cells of the immune system—can determine by themselves the onset rate of aging-associated pathologies, “will help to better understand this process, and it will contribute to the design of strategies to regulate its action. For instance, using the immune receptor CD300f as a target in biomedicine,” notes the expert. “Also, our team has previously shown that some variants of the CD300f immune receptor could be useful as biomarkers in patients.”
The paper, whose first author is the expert Frances Evans (Institute Pasteur and Udelar), includes the participation of teams from the Molecular Imaging Uruguayan Center (CUDIM), among other institutions.
What is the role of this receptor in aging?
The CD300f receptor is a protein expressed by immune system cells that modulates cell metabolism and inflammation. This study reveals the first evidence of its role in the processes related to aging and senescence.
“In particular, we discovered that mice that lacked the CD300f immune receptor developed prematurely some pathologies associated with aging (cognitive deficits, motor incoordination, tumors, etc.) and even damage in several organs such as the brain, the liver or the lungs. Moreover, we observed an important effect on females, the most affected ones,” says Hugo Peluffo.
The study is based on detailed monitoring of several cohorts of animals for thirty months, a methodological innovation that allowed the researchers to see the process of real aging in these animals without using accelerated aging models, which do not fully represent a process that necessarily involves the gradual accumulation of changes with age.
The researcher points out that “the aim is to keep studying the consequences of the dysfunction of the CD300f immune receptor on brain aging, in particular on microglia.”
More information:
Frances Evans et al, CD300f immune receptor contributes to healthy aging by regulating inflammaging, metabolism, and cognitive decline, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113269
Journal information:
Cell Reports
Source: Read Full Article