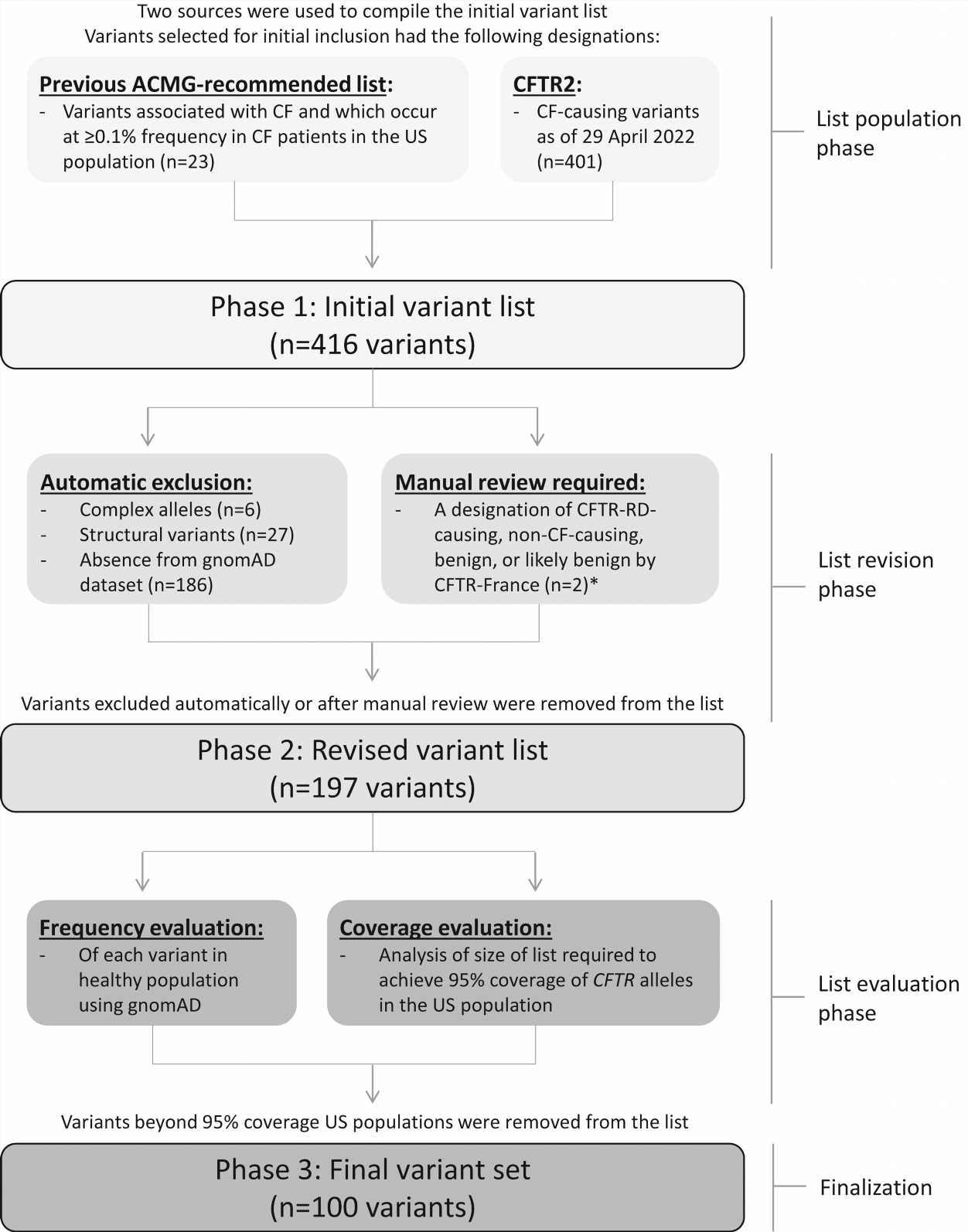
![Flowchart depicting variants for consideration and final inclusion in the updated minimum variant set. All variant nomenclature is based on MANE Select transcript NM_000492.4. Variants for consideration were initially drawn from the previous ACMG-23 variant set and CF-causing variants available on https://cftr2.org as of April 29, 2022. The list was revised by excluding complex alleles, structural variants, and those not present in gnomAD. The remaining variants were evaluated to select those that would collectively achieve 95% coverage of CFTR alleles in the US population. ∗Two variants (c.350G>A [p.Arg117His; legacy: R117H] and c.1013C>T [p.Thr338Ile; legacy: T338I]) deemed CF-causing by CFTR2 have been interpreted as CFTR-RD-causing by CFTR-France and were manually reviewed for pathogenicity; both variants remained for inclusion after manual review. ACMG, American College of Medical Genetics and Genomics; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator. Credit: Genetics in Medicine (2023). DOI: 10.1016/j.gim.2023.100867 Which CFTR variants should be tested by laboratories? New ACMG recommendations](https://scx1.b-cdn.net/csz/news/800a/2023/which-cftr-variants-sh.jpg)
The American College of Medical Genetics and Genomics (ACMG) has released updated recommendations for CFTR carrier screening. Pathogenic variants in the CFTR gene can cause cystic fibrosis (CF) as well as CF-related disorders. The new updated ACMG CFTR variant list includes a set of 100 variants.
The new CFTR variant list represents an updated minimum recommended variant set for CF carrier screening and supersedes the previous group of 23 CFTR variants recommended by the ACMG. These revised recommendations apply to carrier screening, a type of genetic testing used to determine whether a person possesses a genetic variant associated with a condition that typically requires the presence of two pathogenic variants in order to manifest a phenotype.
These revised recommendations do not apply to CFTR variant testing for diagnosis or newborn screening. All other aspects of the updated 2020 ACMG CFTR technical standards still apply.
“When it was originally developed, the previous variant list set the standard for CF carrier screening in the country. Now that our databases and technologies have evolved, it was time to raise the bar and set a new minimum standard. This new recommended variant set should help ensure that CFTR variant detection is more equitable among individuals representing a variety of biogeographic ancestries,” said lead author, Joshua L. Deignan, Ph.D., FACMG.
The updated minimum variant set for CF carrier screening is based on evidence that the variant has been established as CF-causing and is present in the Genome Aggregation Database (gnomAD), the largest and most widely used publicly available collection of population variation from harmonized sequencing data.
For this 2023 version, a conservative approach was used with a framework that only incorporates well-established pathogenic and likely pathogenic variants to minimize concerns that individuals would make reproductive decisions based on limited information. Future versions of this minimum variant set should reassess the feasibility and utility of incorporating additional information from other population databases to be as biogeographically diverse as possible.
History of ACMG’s CFTR carrier screening recommendations
The ACMG has long been involved in addressing the topic of CFTR carrier screening. In 2001, several professional organizations joined in acknowledging the importance and technologic advances that would make CF amenable to population-based carrier screening. However, the technology and knowledge had not advanced far enough to allow for an equitable application.
Sequencing technology was also early in development. This limited screening applied to just small sets of variants that were most commonly characterized in Ashkenazi Jewish and Northern European populations. For this reason, recommendations at that time were that screening should be “offered” to those of Ashkenazi Jewish and Northern European descent and “made available” to other groups.
The ACMG ultimately recommended a set of 25 disease-causing variants, later reduced to 23 to represent a minimum variant set for pan-ethnic carrier screening of individuals with no family history of CF. This minimum variant set (often referred to as the “ACMG-23”) remained unchanged since then, even as molecular diagnostic technologies and genetic knowledge have dramatically advanced.
The original recommendation allowed the option for laboratories to offer an expanded CFTR variant set beyond the recommended set and, at the time, expanded variant sets were met with some controversy. However, several aspects have now evolved, including the widespread availability of cost-effective, high-throughput DNA sequencing as well as more standardized variant classification and interpretation.
In 2020, the ACMG published an updated set of technical standards for CFTR variant testing which recommended that laboratories could now use either targeted or comprehensive methods for testing and at the time reaffirmed the original set of 23 variants as the minimum set for CF carrier screening.
In 2021, the ACMG published a new carrier screening clinical practice resource which continued to recommended offering testing of CFTR (now along with many additional genes) to all pregnant individuals as well as those planning a pregnancy.
More information:
Joshua L. Deignan et al, Updated recommendations for CFTR carrier screening: A position statement of the American College of Medical Genetics and Genomics (ACMG), Genetics in Medicine (2023). DOI: 10.1016/j.gim.2023.100867
Journal information:
Genetics in Medicine
Source: Read Full Article