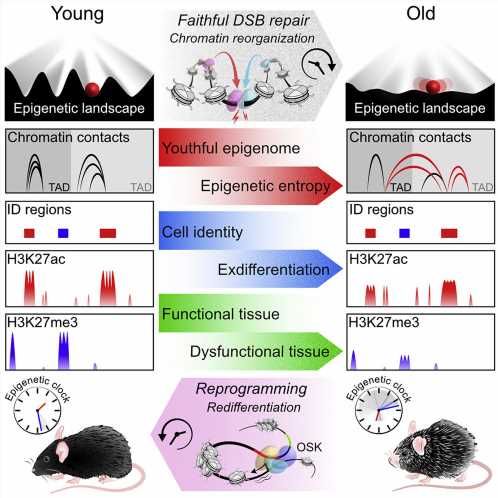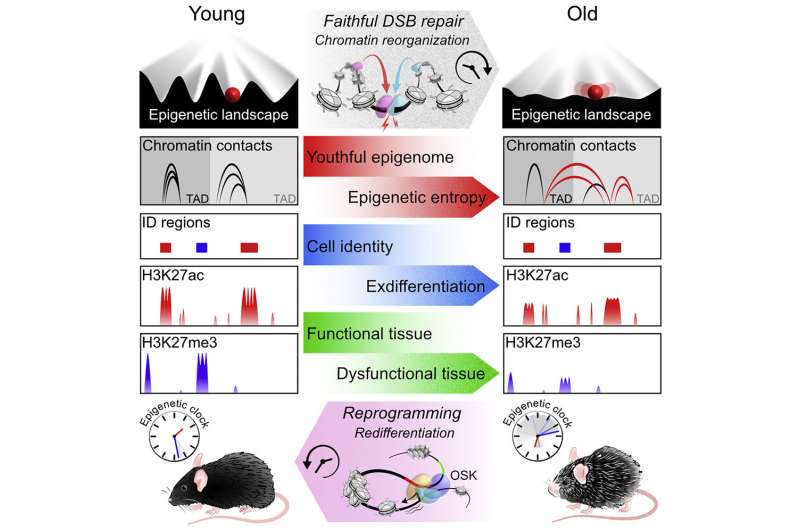
An international study 13 years in the making demonstrates for the first time that degradation in the way DNA is organized and regulated—known as epigenetics—can drive aging in an organism, independently of changes to the genetic code itself.
The work shows that a breakdown in epigenetic information causes mice to age and that restoring the integrity of the epigenome reverses those signs of aging.
Findings are published online Jan. 12 in Cell.
“We believe ours is the first study to show epigenetic change as a primary driver of aging in mammals,” said the paper’s senior author, David Sinclair, professor of genetics in the Blavatnik Institute at Harvard Medical School and co-director of the Paul F. Glenn Center for Biology of Aging Research.
The team’s extensive series of experiments provide long-awaited confirmation that DNA changes are not the only—or even the main—cause of aging. Rather, the findings show, chemical and structural changes to chromatin—the complex of DNA and proteins that forms chromosomes—fuel aging without altering the genetic code itself.
“We expect the findings will transform the way we view the process of aging and the way we approach the treatment of diseases associated with aging,” said co-first author Jae-Hyun Yang, research fellow in genetics in the Sinclair lab.
The authors say that because it’s easier to manipulate the molecules that control epigenetic processes than to reverse DNA mutations, the work points to new avenues that focus on epigenetics rather than genetics to prevent or treat age-related damage.
First, the results need to be replicated in larger mammals and in humans. Studies in nonhuman primates are currently underway.
“We hope these results are seen as a turning point in our ability to control aging,” said Sinclair. “This is the first study showing that we can have precise control of the biological age of a complex animal; that we can drive it forwards and backwards at will.”
Beyond mutations
Perhaps the most burning question for those who study aging is what causes it.
For decades, a reigning theory in the field was that aging arises from an accumulation of changes to DNA, primarily genetic mutations, which over time prevent more and more genes from functioning properly. These malfunctions, in turn, cause cells to lose their identity, so that tissues and organs break down, leading to disease and ultimately death.
In recent years, however, studies have increasingly hinted that there’s more to the story.
For instance, some researchers found that some people and mice with high mutation rates don’t show signs of premature aging. Others observed that many types of aged cells have few or no mutations.
Researchers started wondering what else works with or instead of DNA changes to cause aging. A list of possible culprits grew. Among them were epigenetic changes.
A component of epigenetics is the physical structures such as histones that bundle DNA into tightly compacted chromatin and unspool portions of that DNA when needed. Genes are inaccessible when they’re bundled up but available to be copied and used to produce proteins when they’re unspooled. Thus, epigenetic factors regulate which genes are active or inactive in any given cell at any given time.
By acting as a toggle for gene activity, these epigenetic molecules help define cell type and function. Since each cell in an organism has basically the same DNA, it’s the on-off switching of particular genes that differentiates a nerve cell from a muscle cell from a lung cell.
“Epigenetics is like a cell’s operating system, telling it how to use the same genetic material differently,” said Yang, who is co-first author with Motoshi Hayano, a former postdoctoral fellow in the Sinclair lab who is now at Keio University School of Medicine in Tokyo.
In the late 1990s and early 2000s, Sinclair’s lab and others showed in yeast and mammals that epigenetic changes accompany aging. Yet they couldn’t tell whether these changes drove aging or were a consequence of it.
It wasn’t until the current study that Sinclair’s team was able to disentangle epigenetic from genetic changes and confirm that a breakdown in epigenetic information does, in fact, contribute to aging in mice.
ICE mice
The team’s main experiment involved creating temporary, fast-healing cuts in the DNA of lab mice.
These breaks mimicked the low-grade, ongoing breaks in chromosomes that mammalian cells experience every day in response to things like breathing, exposure to sunlight and cosmic rays, and contact with certain chemicals.
In the study, to test whether aging results from this process, the researchers sped the number of breaks to simulate life on fast-forward.
The team also ensured that most of the breaks were not made within the coding regions of the mice’s DNA—the segments that make up genes. This prevented the animals’ genes from developing mutations. Instead, the breaks altered the way DNA is folded.
Sinclair and colleagues called their system ICE, short for inducible changes to the epigenome.
At first, epigenetic factors paused their normal job of regulating genes and moved to the DNA breaks to coordinate repairs. Afterward, the factors returned to their original locations.
But as time passed, things changed. The researchers noticed that these factors got “distracted” and did not return home after repairing breaks. The epigenome grew disorganized and began to lose its original information. Chromatin got condensed and unspooled in the wrong patterns, a hallmark of epigenetic malfunction.
As the mice lost their youthful epigenetic function, they began to look and act old. The researchers saw a rise in biomarkers that indicate aging. Cells lost their identities as, for example, muscle or skin cells. Tissue function faltered. Organs failed.
The team used a recent tool developed by Sinclair’s lab to measure how old the mice were, not chronologically, in days or months, but “biologically,” based on how many sites across the genome lost the methyl groups normally attached to them. Compared to untreated mice born at the same time, the ICE mice had aged significantly more.
Young again
Next, the researchers gave the mice a gene therapy that reversed the epigenetic changes they’d caused.
“It’s like rebooting a malfunctioning computer,” said Sinclair.
The therapy delivered a trio of genes—Oct4, Sox2, and Klf4, together named OSK—that are active in stem cells and can help rewind mature cells to an earlier state. (Sinclair’s lab used this cocktail to restore sight in blind mice in 2020.)
The ICE mice’s organs and tissues resumed a youthful state.
The therapy “set in motion an epigenetic program that led cells to restore the epigenetic information they had when they were young,” said Sinclair. “It’s a permanent reset.”
How exactly OSK treatment achieved that remains unclear.
At this stage, Sinclair says the discovery supports the hypothesis that mammalian cells maintain a kind of backup copy of epigenetic software, that when accessed, can allow an aged, epigenetically scrambled cell to reboot into a youthful, healthy state.
For now, the extensive experiments led the team to conclude that “by manipulating the epigenome, aging can be driven forwards and backwards,” said Yang.
From here
The ICE method offers researchers a new way to explore the role of epigenetics in aging and other biological processes.
Because signs of aging developed in the ICE mice after only six months rather than toward the end of the average mouse life span of two and a half years, the approach also saves time and money for researchers studying aging.
Researchers can also look beyond OSK gene therapy in exploring how lost epigenetic information might be restored in aged organisms.
“There are other ways to manipulate the epigenome, like drugs and small molecule chemicals that induce gentle stress,” said Yang. “This work opens a door for applying those other methods to rejuvenate cells and tissues.”
Sinclair hopes the work inspires other scientists to study how to control aging to prevent and eliminate age-related diseases and conditions in humans, such as cardiovascular disease, type 2 diabetes, neurodegeneration, and frailty.
“These are all manifestations of aging that we’ve been trying to treat with medicines when they arise, which is almost too late,” he said.
The goal would be to address the root causes of aging to extend human health span: the number of years that a person remains not just alive but well.
Medical applications are a long way off and will take extensive experiments in multiple cell and animal models. But, Sinclair said, scientists should think big and keep trying in order to achieve such dreams.
“We’re talking about taking someone who’s old or sick and making their whole body or a specific organ young again, so the disease goes away,” he said. “It’s a big idea. It’s not how we typically do medicine.”
More information:
Jae-Hyun Yang et al, Loss of epigenetic information as a cause of mammalian aging, Cell (2023). DOI: 10.1016/j.cell.2022.12.027
Journal information:
Cell
Source: Read Full Article