When adult humans and animals severely injure their spinal cord, they become irreversibly paralyzed and are no longer able to move body parts controlled by the section of the spinal cord below their lesion. To counteract the damage done by the injury, doctors often use pharmacological agents or electrical stimulation tools.
While these treatments can have some positive effects, they only enable movements temporarily and do not restore full mobility in the long term. Interestingly, studies on rodents have showed that while severe spinal cord injuries cause permanent paralysis in adults, neonatal rodents with spinal cord lesions can recover much of their hindlimb mobility in adulthood, without receiving any targeted treatments.
Neuroscientists have been trying to understand the biological mechanisms underlying this renewed mobility for some years now. However, the reasons why the same type of injury experienced at different ages can have different long-term consequences on mobility remains unclear.
A team of researchers at VIB- Neuroelectrics Research Flanders (NERF) and Leuven Brain Institute have recently carried out a study investigating the mechanisms underlying the recovery of hindlimb mobility in neonatal rodents with spinal lesions. Their findings, published in Nature Neuroscience, suggest that this recovery could be regulated by the neurotransmitter phenotype switching of spinal excitatory interneurons.
In their experiments, the researchers subjected a series of wild mice to a complete spinal cord transection in the lower part of their thorax. The mice they operated on were either adults or five days old.
Subsequently, the researchers used high-speed cameras to observe and characterize the movements of the mice as they moved on a treadmill, comparing them to those of a control group of mice (i.e., mice with no spinal lesions). Finally, the researchers carried out a series of analyses to determine the biological and kinematic differences between the adult and post-natal mice with spinal cord injuries.
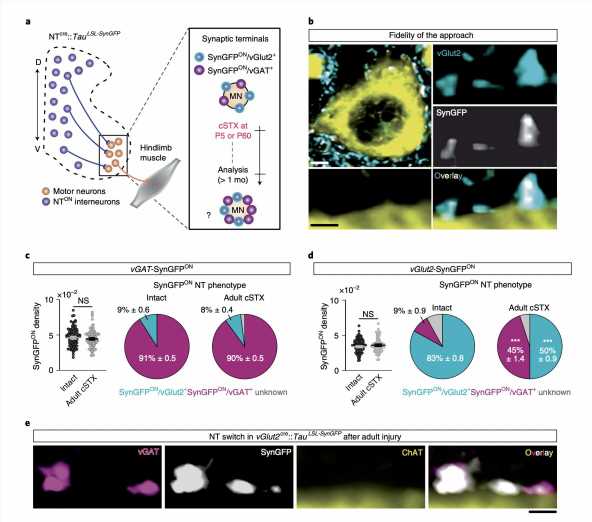
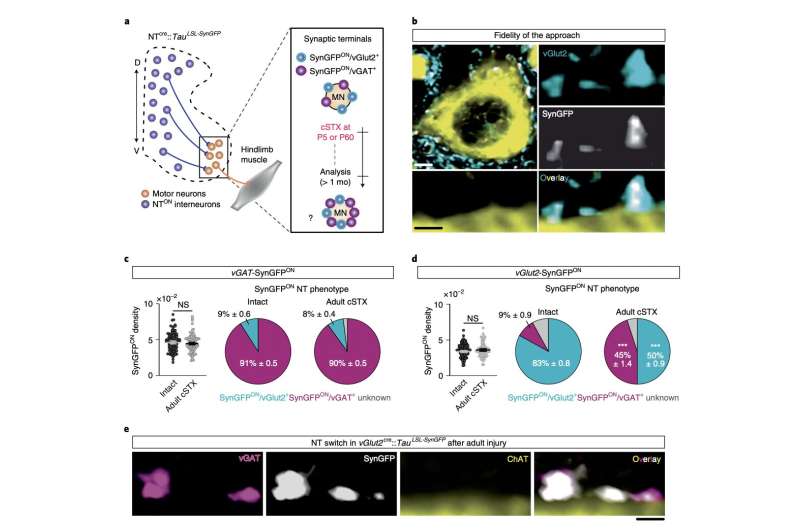
“We found that adult spinal cord injury prompts neurotransmitter switching of spatially defined excitatory interneurons to an inhibitory phenotype, promoting inhibition at synapses contacting motor neurons,” Hannah Bertels, Guillem Vicente-Ortiz, Khadija El Kanbi, and Aya Takeoka wrote in their paper. “In contrast, neonatal spinal cord injury maintains the excitatory phenotype of glitamatergic interneurons and causes synaptic sprouting to facilitate excitation.”
Essentially, the researchers’ findings suggest that the recovery of hindlimb mobility in post-natal mice with spinal cord injuries is regulated by the switching of injury-specific neurotransmitter identities and the rearrangements of connectivity among excitatory interneurons. Excitatory interneurons are neurons that connect spinal motor and sensory neurons, which utilize the neurotransmitter glutamine.
Bertels and her colleagues then performed a series of further tests aimed at understanding their observations more in depth. The findings they collected offer valuable insight that could pave the way for new studies focusing on the consequences of severe spinal cord injuries.
“Genetic manipulation to mimic the inhibitory phenotype observed in excitatory interneurons after adult spinal cord injury abrogates autonomous locomotor functionality in neonatally injured mice,” the researchers added in their paper. “In comparison, attenuating this inhibitory phenotype improves locomotor capacity after adult injury. Together, this data demonstrates that neurotransmitter phenotype of define excitatory interneurons steers locomotor recovery after spinal cord injury.”
Source: Read Full Article