In a recent article published in the journal Proceedings of the National Academy of Sciences (PNAS), researchers reviewed the present-day therapeutic landscape of coronavirus disease 2019 (COVID-19) and addressed future opportunities to overcome challenges that hinder drug development and improve the global preparedness for future pandemics.
The unprecedented morbidity and mortality due to COVID-19 have been a global concern. The relentless work of researchers, combined with huge investments made by national and international agencies, has facilitated the development of vaccines and therapeutic drugs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Although the administration of repurposed drugs has decreased COVID-19 mortality, there is a large proportion of the global population that continues to remain at risk. Moreover, while a few therapeutic drugs such as hydroxychloroquine have been extensively investigated, other drugs such as those reducing granulocyte-macrophage colony-stimulating factor (anti–GM-CSF) or tumor necrosis factor (anti-TNF) have not been evaluated at a large scale to date. Therefore, a standardized drug regimen for COVID-19 is lacking.
Present COVID-19 therapeutics and their limitations
The present-day therapeutic landscape of COVID-19 involves antivirals, monoclonal antibodies (mAbs), anti-inflammatory agents, anticoagulants, and inhaled therapeutics. Antiviral agents such as polymerase inhibitors (remdesivir and molnupiravir) and protease inhibitors (3CLpro and ritonavir) have been effective in early COVID-19. However, their efficacy in asymptomatic COVID-19 patients is questionable.
Combinational monoclonal antibodies (mAbs) such as bamlanivimab-etesevimab are highly specific and, thus, less toxic but have reduced pan-variant efficacy. Contrastingly, sotrovimab, which targets a preserved SARS-CoV-2 epitope has demonstrated sustained efficacy across viral variants. However, their intravenous route of administration hinders their widespread use. Overall, low availability, high costs, and complex logistics have limited extensive use of mAbs.
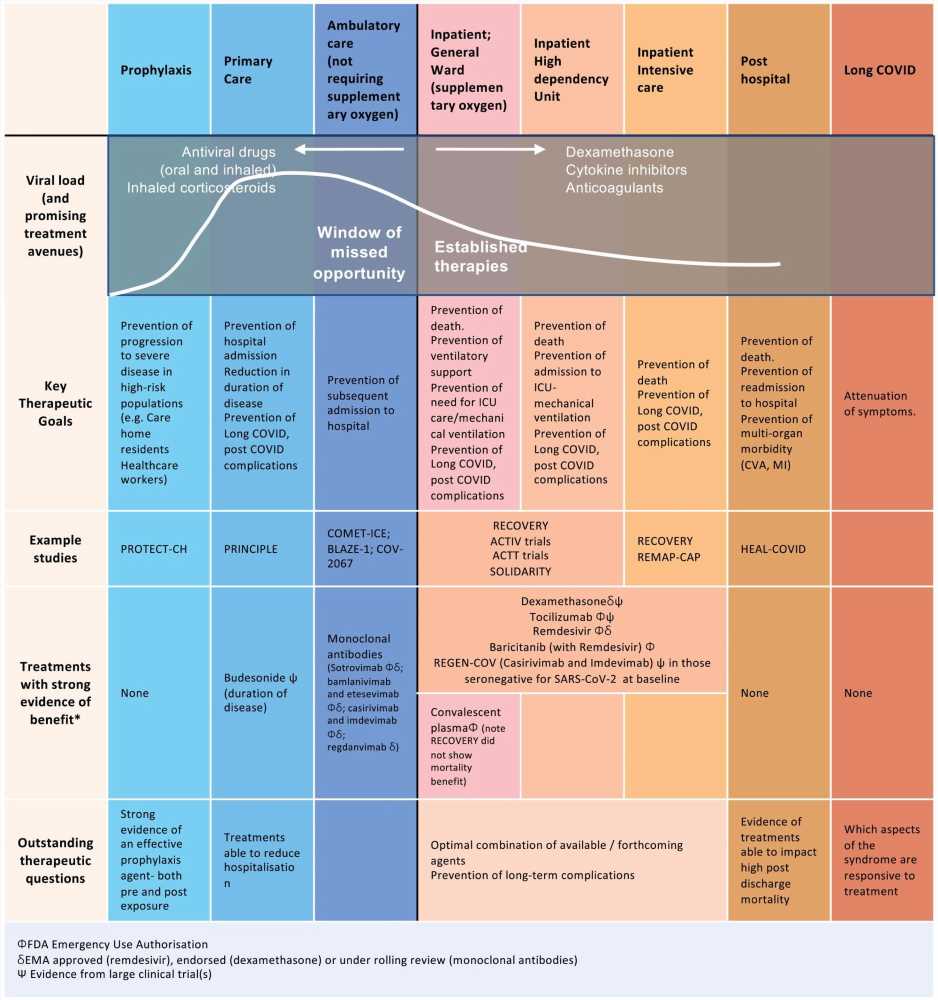
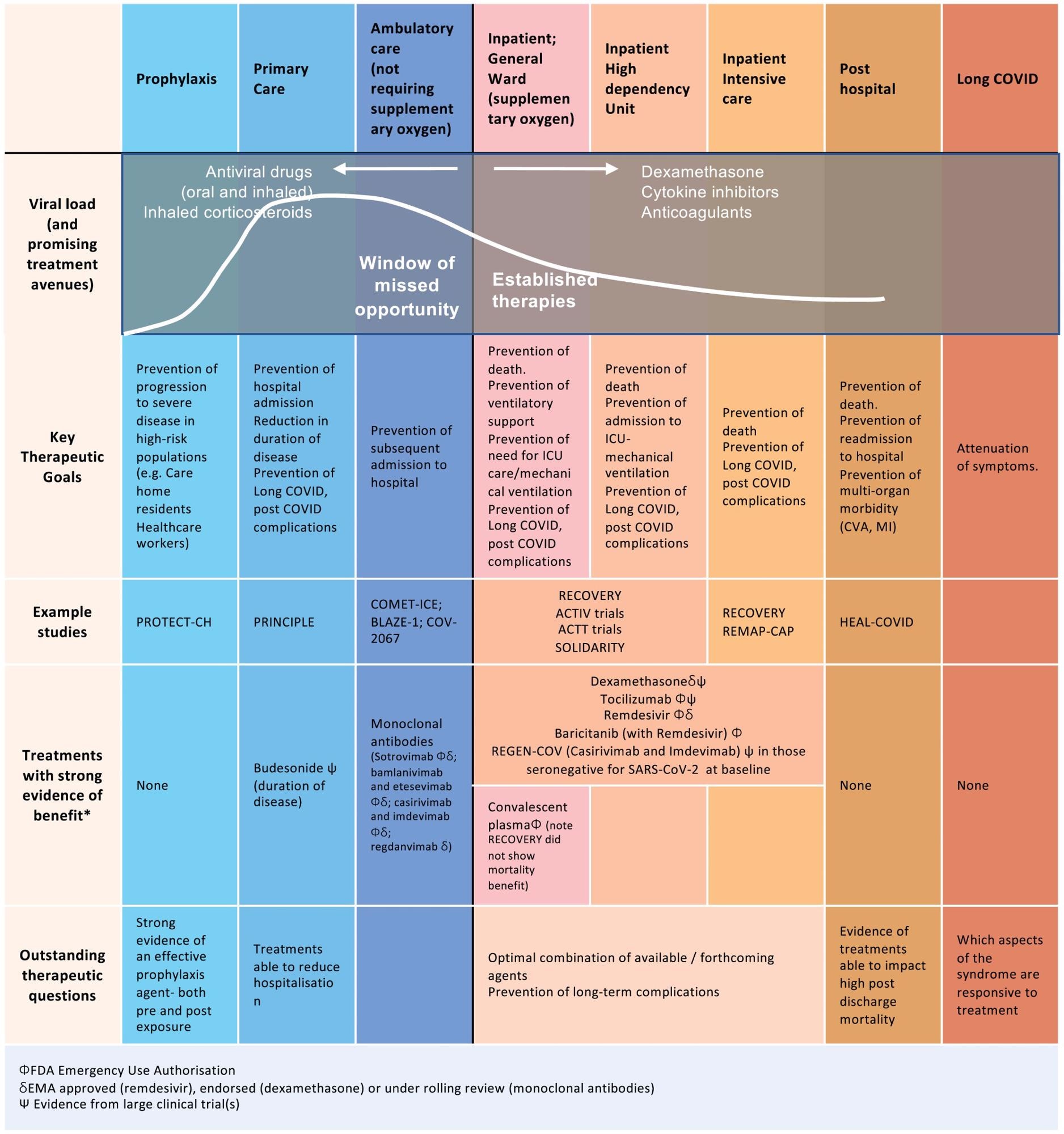 Established therapies and future opportunities for intervention early in disease. Following infection with SARS-CoV-2, there are specific time points in the disease trajectory where different therapies could be optimally administered. At this time, the majority of treatments have been targeted during hospitalization and particularly at late stages of acute disease during ICU admission. Additionally, many of the therapies trialed are antibody therapies and are cost-prohibitive, especially in low- to middle-income countries. There is currently a window of missed opportunity early in disease to reduce progression to hospitalization.
Established therapies and future opportunities for intervention early in disease. Following infection with SARS-CoV-2, there are specific time points in the disease trajectory where different therapies could be optimally administered. At this time, the majority of treatments have been targeted during hospitalization and particularly at late stages of acute disease during ICU admission. Additionally, many of the therapies trialed are antibody therapies and are cost-prohibitive, especially in low- to middle-income countries. There is currently a window of missed opportunity early in disease to reduce progression to hospitalization.
COVID-19 has been characterized by an excessive elevation in cytokine levels such as interleukins (IL)-1,6, GM-CSF, TNF, interferon-alpha–inducible protein 10 (IP-10), and monocyte chemoattractant protein 1a (MCP1). Thus, anti-inflammatory agents such as corticosteroids have benefitted COVID-19 patients, especially those with severe disease. The IL-6 levels have correlated with viral loads and COVID-19 severity. Thus, anti-IL-6 agents such as Tocilizumab have decreased COVID-19 severity when used in conjunction with glucocorticoids.
Angiotensin-converting enzyme 2 (ACE2)-mediated thrombosis has been noted in pulmonary and extrapulmonary vasculature in COVID-19. Thus, anticoagulants such as low molecular weight heparin have been routinely used. However, given the interplay of the immune system and coagulation pathways, combination regimens of anti-inflammatory agents and anticoagulants could be more effective.
Novel inhalation therapies such as inhaled corticosteroids (ICS) and inhaled IFN-β therapies are cost-effective, easily administrable, and effective in early COVID-19 with minimal side effects. ICS down-regulate ACE2 expression and therefore restrict viral entry into host cells.
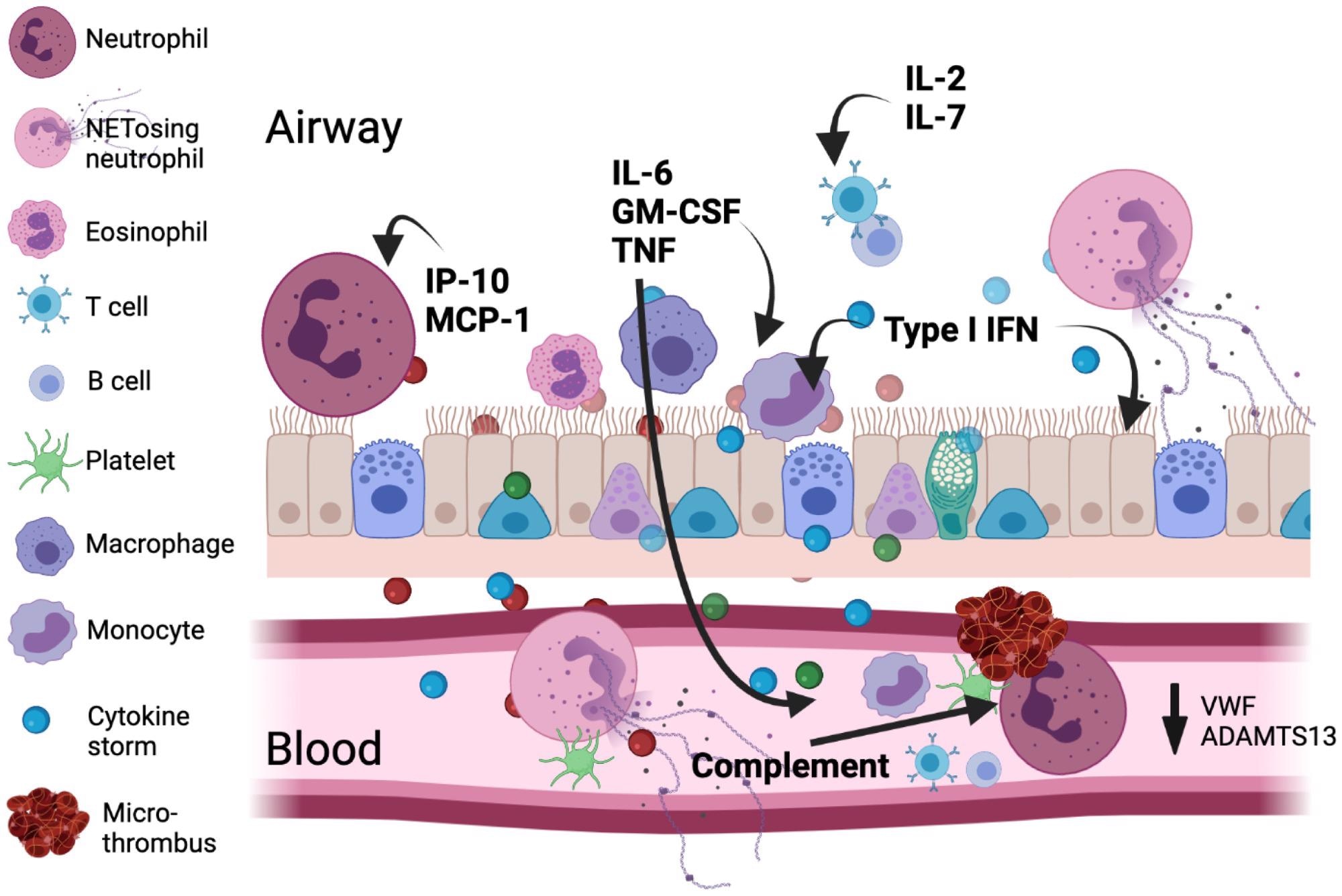 Major immunologic and coagulatory factors are implicated in COVID-19 pathology. The viral infection leads to type I IFN, inflammatory mediator, and alarmin release from the respiratory epithelium/endothelium and resident immune cells setting up a chemotactic gradient pulling cells from the circulation into the lung. An emergency myelopoietic state occurs and neutrophils and monocytes display abnormalities in the blood in this state of high inflammation. Lymphocytes concurrently become depleted in circulation. Activated monocytes and macrophages can be an important source of cytokines, including IL-6. Enhanced by complement, clusters of neutrophils and activated platelets occur and neutrophil NETosis in blood and tissue directly augments thrombosis by supporting platelet activation. The size for each cell indicates its relative abundance in each compartment.
Major immunologic and coagulatory factors are implicated in COVID-19 pathology. The viral infection leads to type I IFN, inflammatory mediator, and alarmin release from the respiratory epithelium/endothelium and resident immune cells setting up a chemotactic gradient pulling cells from the circulation into the lung. An emergency myelopoietic state occurs and neutrophils and monocytes display abnormalities in the blood in this state of high inflammation. Lymphocytes concurrently become depleted in circulation. Activated monocytes and macrophages can be an important source of cytokines, including IL-6. Enhanced by complement, clusters of neutrophils and activated platelets occur and neutrophil NETosis in blood and tissue directly augments thrombosis by supporting platelet activation. The size for each cell indicates its relative abundance in each compartment.
Future perspective
Several antivirals targeting SARS-CoV-2 proteins such as polymerase, papain-like protease, helicase, and viral replication transcription complexes (RTC) responsible for viral ribonucleic acid (RNA) synthesis, proofreading, and 5’ -capping are under research. Additionally, drug combinations such as remdesivir-recombinant soluble ACE2 that differently target the SARS-CoV-2 life cycle have been effective in vitro.
Drug efficacy is usually determined using cultured cell lines. However, the cell lines used vary among trials, yielding non-standardized results. Thus, future trials must incorporate uniform cell lines to develop antivirals with pan-virus and pan-variant efficacy.
Host-targeted therapeutics include enzymes of glycan-mediated endoplasmic reticulum quality control (ERQC) for the folding of viral glycoproteins. Iminosugars, such as Miglustat and MON-DNJ, that inhibit ERQC enzymes can be easily administered orally and are cost-effective. Thus, they must be researched further to enable swift clinical translation. Iminosugars may reduce not only the epidemic potential but also delay the emergence of new viral variants.
Surprisingly, anti-TNF agents that are commonly used in several autoimmune conditions have not been explored in COVID-19 to date. MCP-1 and IP-10 may be promising candidates since their levels have correlated with COVID-19 severity. Furthermore, agents targeting neutrophilia and monocyte dysfunction in COVID-19 need to be investigated. Thus, lofty investments must be made to facilitate scientific research.
Novel agents such as deoxyribonucleic acid (DNA) aptamers could be delivered intranasally. In addition, combinations of ICS and IFN-β should be explored for their efficacy. Aptamer manufacture has several advantages over antibodies, such as lower cost, no cold chain logistics, and can be inhaled.
Further, immunological biomarkers and machine learning must be used to identify the highest priority individuals for COVID-19 therapy. Although remdesivir and Tocilizumab are established agents for hospitalized COVID-19 patients, the high costs preclude their use in the developing world.
The speed of clinical trials can be ramped up by using adaptive platforms, routinely collected data as primary data, and improved use of existing data linkage tools. Adaptive platforms allow ineffective interventions to be discarded and new candidates to be introduced. Additionally, collaborative efforts among trial teams, drug manufacturers, managers, and regulators and easy availability of accurate surrogate marker-based data from previous trials are required to accelerate subsequent trials.
Overall, the authors suggested that drugs must be tailored to the stage of COVID-19, and an in-depth understanding of SARS-CoV-2 pathogenesis is required with the use of high-resolution imaging to elucidate the pathogenic changes and develop effective drugs. The drugs must be cost-effective, easily administrable, highly efficacious, and widely accessible for global use. Moreover, data accessibility, adaptive platforms, and collaborative efforts are required to streamline therapeutic research.
- COVID-19 therapeutics: Challenges and directions for the future. Philip C. Robinson, David F. L. Liew, Helen L. Tanner, John R. Grainger, Raymond A. Dwek, Ronald B. Reisler, Lawrence Steinman, Marc Feldmann, Ling-Pei Ho, Tracy Hussell, Paul Moss, Duncan Richards, and Nicole Zitzmann. PNAS 2022 Vol. 119 No. 15 e2119893119, DOI: https://doi.org/10.1073/pnas.2119893119, https://www.pnas.org/doi/10.1073/pnas.2119893119
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Anti-Inflammatory, Aptamers, Blood, Cell, Cold, Cold chain, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, DNA, Drugs, Efficacy, Enzyme, Glycan, Helicase, Heparin, Hydroxychloroquine, Imaging, Immune System, in vitro, Inflammation, Interferon, Machine Learning, Macrophage, Miglustat, Monocyte, Mortality, Necrosis, Neutrophils, Pathology, Platelet, Platelets, Polymerase, Protein, Remdesivir, Research, Respiratory, Ribonucleic Acid, Ritonavir, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Sotrovimab, Syndrome, Therapeutics, Thrombosis, Transcription, Translation, Tumor, Tumor Necrosis Factor, Vasculature, Virus

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article