FDA Denies Approval for Patisiran in ATTR Cardiomyopathy
The US Food and Drug Administration (FDA) has declined to approve the RNA interference (RNAi) therapeutic agent patisiran (Onpattro, Alnylam Pharmaceuticals) for treatment of transthyretin-mediated (ATTR) amyloidosis with cardiomyopathy, the company has announced. ATTR amyloidosis is an underdiagnosed, rapidly progressive,…
Read MoreNonsurgical treatment shows advantages in Peyronies disease
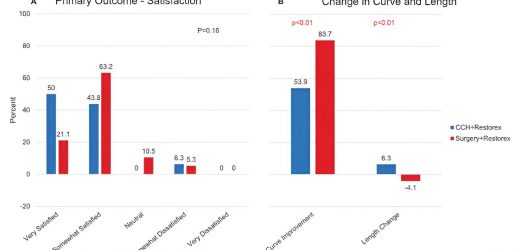
For men with Peyronie’s disease (PD), nonsurgical treatment including injections of collagenase clostridium histolyticum (CCH) produces high satisfaction with sexual outcomes—with fewer adverse events compared to surgery. These are the results of a clinical trial reported in the October issue…
Read MoreBetter benefits from home-based walking than supervised facility exercise in PAD patients
Research led by the Feinberg School of Medicine, Northwestern University, Illinois, found that home-based walking exercise regimens outperformed supervised treadmill physical therapy in patients with lower extremity peripheral artery disease. In a paper, “Home-Based Walking Exercise and Supervised Treadmill Exercise…
Read MoreSmart scalpel could help doctors hone surgical skills
Sensor-loaded device Surgical procedure Assessing skills Source: Read Full Article
Read MoreMajor study involving centenarians may have found the key to longevity
Loose Women: Dr Hilary discusses how to live longer If you could know the future, the answer to how long you’ll live would probably rank high on the list of questions. While fortune telling isn’t usually very accurate, your body…
Read MoreInternational team of scientists says identifying some foods as addictive could shift attitudes, stimulate research
Researchers from the United States, Brazil, and Spain, including scientists with the Fralin Biomedical Research Institute at VTC, published an analysis in a special edition of the British Medical Journal (BMJ ) with a timely and controversial recommendation: It’s time…
Read MoreDietary Changes to Microbiome May Improve Lung Function
HONOLULU — What we eat and what’s in the gut may influence lung health for better or worse, suggest new data from an ongoing study of lung function in New York City firefighters who were at the World Trade Center…
Read MoreThe middle-aged cocaine abusers creating a timebomb for the NHS
From heart attacks to dementia, the middle-aged cocaine abusers creating a timebomb for the NHS Figures show over-40s are the prime victims of recreational drug damage Being hospitalised or dying from taking illegal drugs such as cocaine is something we…
Read MoreLoving parent-child relationships boost prosociality and mental health
Reviewed A loving bond between parents and their children early in life significantly increases the child's tendency to be 'prosocial', and act with kindness and empathy towards others, research indicates. The University of Cambridge study used data from more than…
Read More