Vision allows brain to make predictions well before it knows whats coming, new study shows
The moment a pitcher unleashes a fastball in the direction of Toronto Blue Jays shortstop Bo Bichette in a professional baseball game, the crowd at Rogers Centre hopes something special is about to happen. But while they can’t predict it,…
Read MoreParents, caregivers generally support mental health screening
Most parents and caregivers support mental health screening for their child, according to a study published online June 20 in JAMA Network Open. Mirelle Kass, from the Child Mind Institute in New York City, and colleagues examined parents’ and caregivers’…
Read MoreAmazon Clinic may delay its nationwide telehealth launch
Amazon Clinic, with subscription-based telehealth services in 33 states, appears to be holding off on a promotional campaign announcing its nationwide expansion that was reportedly planned to launch today because of a letter asking about the telemedicine platform’s patient privacy…
Read MoreMyasthenia Gravis Drug Gets FDA Nod
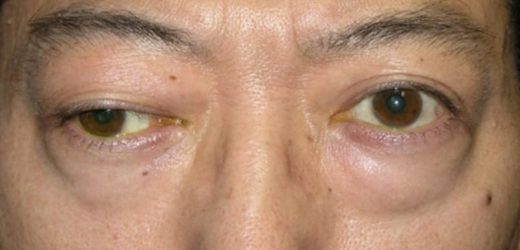
The US Food and Drug Administration (FDA) has approved rozanolixizumab (Rystiggo) to treat adults with generalized myasthenia gravis (gMG) who are positive for anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody, the drug’s manufacturer, UCB, has announced. gMG is…
Read MoreHairy findings: chemicals study jolts French senators
Mercury, pesticides and rare earth metals form a toxic cocktail that a French laboratory announced Tuesday it has found in an unlikely place: on the heads of senators. Twenty-six members of France’s Socialist Party who sit in the upper house…
Read MoreHigher doses of oral semaglutide improves blood sugar control and weight loss
Diabetes is a progressive disease that affects one’s ability to control blood sugar levels. For many patients, the condition becomes more severe over time and blood sugar levels grow more difficult to manage. Glucagon-like peptide-1 (GLP-1) receptor agonists, such as…
Read MoreOnce-Weekly Basal Insulin Nears Market for Type 2 Diabetes
SAN DIEGO – The investigational once-weekly insulin icodec provided superior glucose control compared with the once-daily basal insulins degludec and glargine in type 2 diabetes, results from two new phase 3a studies suggest. Data from Novo Nordisk’s ONWARDS 1, comparing…
Read MoreTeam reports on development of potential new generic treatment for multiple types of cancer
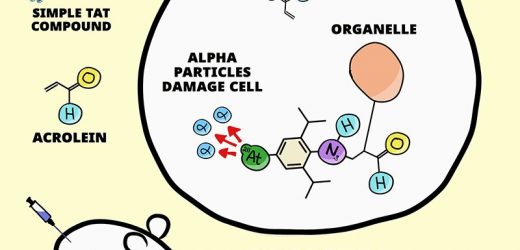
Researchers led by Katsunori Tanaka at the RIKEN Cluster for Pioneering Research (CPR) in Japan and Hiromitsu Haba at the RIKEN Nishina Center for Accelerator-Based Science (RNC) have developed a new technique that has the potential to generically treat several…
Read MoreDoctor explains differences between heat stroke and heat exhaustion
Doctor explains key differences between heat stroke and heat exhaustion Doctor reveals key differences between heat stroke and heat exhaustion Whilst Britain is basking in the sun with more blistering sunshine set to follow, the continued hot weather does have…
Read MoreLean body mass and age predict how quickly women eliminate alcohol
The rate at which women eliminate alcohol from their bloodstream is largely predicted by their lean body mass, although age plays a role, too, scientists found in a new study. Women with obesity – and those who are older –…
Read More