For the millions of people who could face a cancer diagnosis this year, it’s vital to have all therapeutic options available.
But in some cases, the drugs used to treat cancer can cause other health problems during treatment or later. One example is a class of drugs called anthracyclines, which are derived from bacteria found in Italian soil decades ago. This group of about six chemotherapy drugs has been used since the 1960s to treat leukemia, lymphoma, sarcoma and breast cancer, as well as other cancers that have spread from their original site. But a serious side effect of anthracyclines is heart damage either during treatment—which means patients must stop using it—or weeks, months or even years later. This heart damage can decrease the quality and duration of a former cancer patient’s life.
To find the best path for care, a team of clinician-researchers and discovery scientists report on a potential way to prevent or heal heart damage when anthracyclines are used to treat cancer. The paper is published in Circulation Research.
Treatment to toxin
Xiaolei Xu, Ph.D., who directs the Zebrafish Genetics Laboratory at Mayo Clinic and specializes in studying cardiac disease, combined forces with Joerg Herrmann, M.D., director of the Cardio-Oncology Clinic at Mayo Clinic.
“The liaison between basic and clinical science is critical for advancing the medical field in general and especially this field,” says Dr. Herrmann. “Only through new research discoveries at the bench and their translation to the bedside are we able to improve care and outcomes of patients who are in dire need of it.”
Together the researchers are working to uncover the basic mechanisms of anthracycline heart failure in preclinical models and then translate that knowledge into drug treatments for patients.
“We leveraged zebrafish as a more efficient vertebrate model for the initial step of the drug screen,” says Dr. Xu. “A higher number of drugs can be assessed in the zebrafish model, and the top-ranking drugs are also effective in treating anthracycline-induced cardiotoxicity in a mouse model of the disease.”
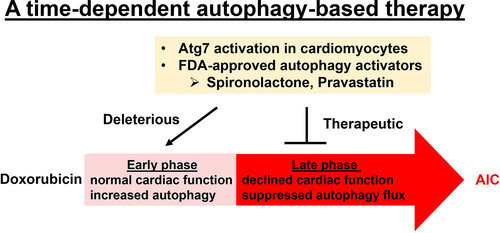
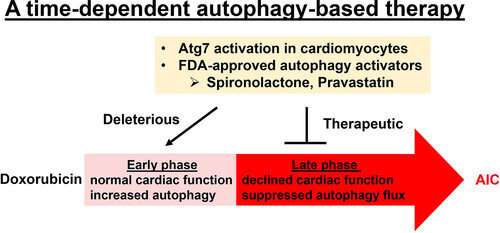
Using an embryonic and then an adult zebrafish model of anthracycline-induced cardiotoxicity with the drug doxorubicin, the researchers identified a key cellular process linked to heart damage. Called “autophagy,” this process recycles and disposes of waste proteins and damaged cellular machinery, such as mitochondria. In zebrafish, researchers could see that soon after treatment with doxorubicin, heart cells increased the process of autophagy. But over time, autophagy decreased, and the heart became dysfunctional. When researchers increased the availability of a protein crucial to autophagy, that process rebounded, and the zebrafish heart function improved.
The scientists also tested medications linked to increasing autophagy and then tried those drugs in a mouse model of anthracycline-induced cardiotoxicity. In all models, the drugs used to boost autophagy increased damage in the early phase of treatment with doxorubicin. But the drugs restored the process of autophagy and thereby not only prevented further cellular damage, but also recovered heart function in the late phase.
“This discovery addresses conflicting results that have been lingering in the anthracycline-induced cardiotoxicity field for many years and could benefit many cancer patients directly,” says Dr. Xu.
From a clinical perspective, Dr. Herrmann says the approach to anthracycline cardiomyopathy has focused largely on prevention, and efforts to improve treatment of the condition are limited.
Source: Read Full Article