The earliest moments of human development—between the time of fertilization and when the embryo implants in the womb—have remained opaque to scientists. Cells in the earliest stages of human development have the ability to generate all tissues in the body and the placenta. Unfortunately, issues such as miscarriage and birth defects can occur, but are not well understood. Until recently, peering into the inner workings of the earliest stages of development has relied on the use of human embryos. But research using human embryos, typically from IVF embryos that fail to meet quality standards, is highly restricted due to ethical concerns.
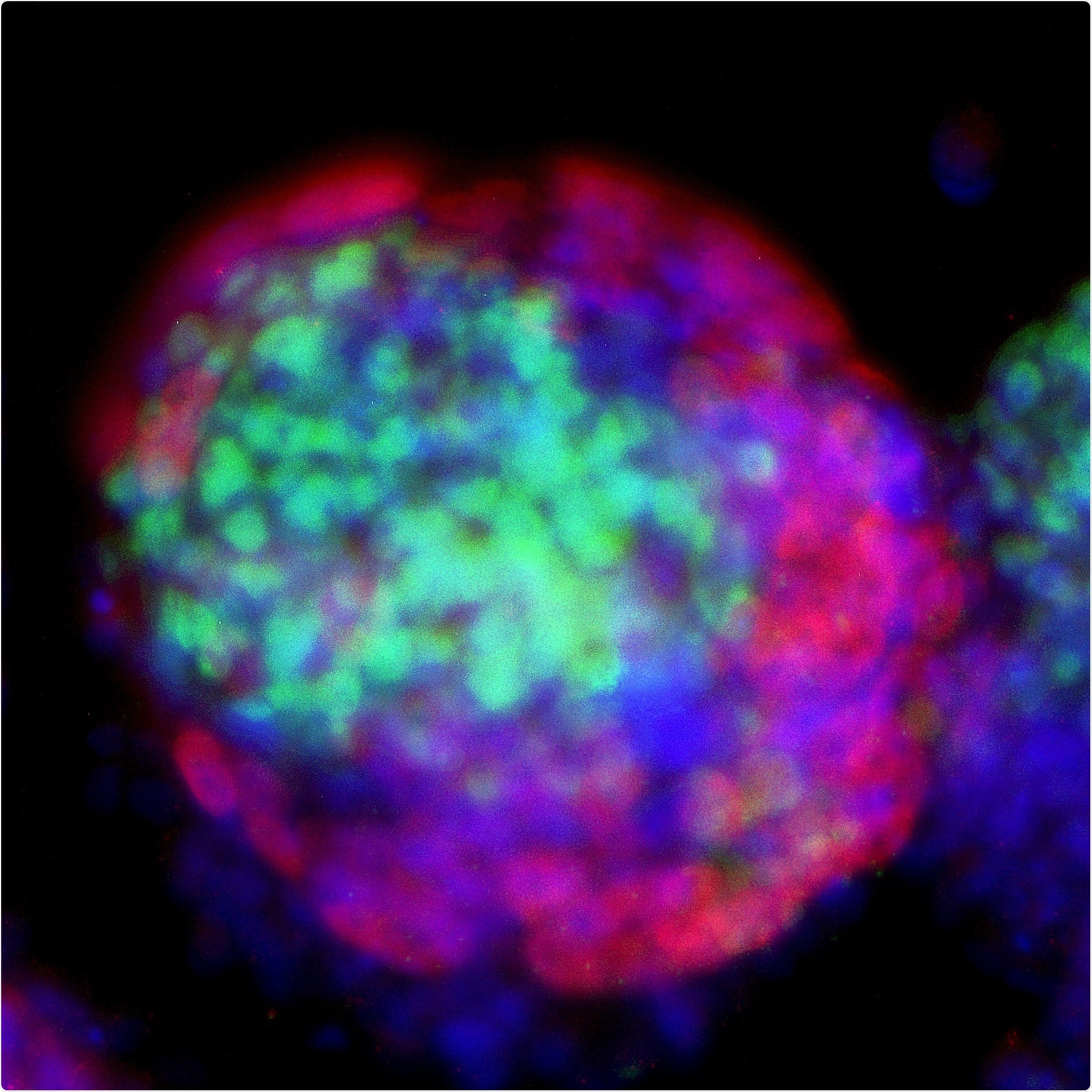
Recently, scientists have found a special type of lab grown stem cell named extended pluripotent stem cells (EPSCs) that have a dual potential to become cells in the embryo and the placenta. These EPSCs raise the possibility of using stem cells to construct a model for studying human pre-implantation embryogenesis without the use of human embryos.
A joint team of researchers from KAUST and Peking University Third Hospital have set out to use EPSCs to make embryo-like 3D structures, from which a better understanding of the earliest embryonic lineages may be obtained. In a paper published today in the journal Cell Discovery KAUST faculty member Mo Li and his co-authors have sought to avoid ethical concerns with the use of tissue from human or mammalian embryos, with the creation of something new.
The team have created a novel model for human development called a blastoid. A blastoid is essentially a cell model of the blastocyst phase of early human development. The key difference being that it is not capable of becoming a viable embryo, and therefore avoids many of the ethical shortcomings of conventional research.
“Our success is built on our previous work on making mouse blastoids using mouse EPSCs,” says Prof. Yang Yu at Peking University Third Hospital, who led the study.
Human blastoids turned out to be much tricker to make, and we had to come up with a new way of exploiting the dual potential of human EPSCs to make them happen.”
Prof. Mo Li, KAUST Faculty Member, Study Co-Leader
The team has showed that blastoids can be cultured further to mimic some features of post-implantation development. For decades a global moratorium has been in place on the cultivation of human embryos beyond the 14-day window, which has severely limited the ability of researchers to map the differentiation of the early cell lineages. This rule may be changing, as the International Society for Stem Cell Research has removed the 14-day limit on in vitro human embryo studies and ignited a public debate. Nonetheless, such studies will be governed by strict ethical reviews and will be still limiting.
Human blastoids are stem cell-based experimental models that do not involve the destruction of human embryos. Scientists and ethicists have argued that blastoids should not be subjected to the same ethical rules as nature embryos. The investigators stopped their blastoid experiments at day 10 to adhere to the 14-day rule. Their future plan is to focus on improving the efficiency of the derivation of blastoids and make them a robust model not only for unlocking the black box that is early human development but also for testing treatments for fertility disorders.
King Abdullah University of Science and Technology
Fan, Y., et al. (2021) Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discovery. doi.org/10.1038/s41421-021-00316-8.
Posted in: Cell Biology
Tags: Birth Defects, Blastocyst, Cell, Embryo, Embryogenesis, Fertility, Fertilization, Hospital, Human Embryos, Implants, in vitro, IVF, Laboratory, Miscarriage, pH, Placenta, Research, Stem Cells, Womb
Source: Read Full Article
