A new drug has shown promise in slowing the regrowth of tumors among some bowel cancer patients, according to new findings of a major trial run by researchers at UCL in collaboration with the Universities of Glasgow, Oxford, Leeds and Cardiff.
The results of the FOCUS4-C trial, which was funded by Cancer Research UK, the EME Program—an MRC/NIHR partnership—and AstraZeneca, was presented on Saturday (18 September) at the European Society of Medical Oncology and published this week in the Journal of Clinical Oncology.
The trial looked at whether a drug called adavosertib, taken in the form of a daily pill, could delay tumor regrowth among patients with an aggressive sub-type of inoperable bowel cancer who have limited treatment options.
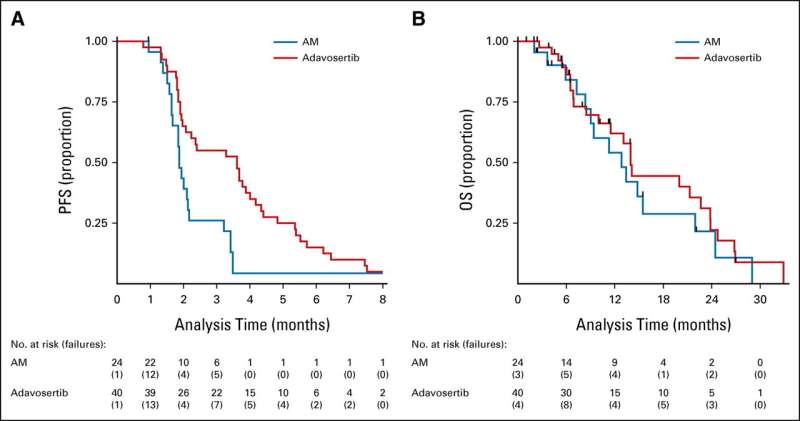
Comparing 44 patients who took adavosertib with 25 patients who did not, the researchers found that the drug delayed tumor growth by about two months on average and had relatively few side effects. The drug had more effect in the 31 patients with left-sided/rectal tumors, increasing overall survival—that is, patients lived longer.
The findings come from one part of a large collaborative UK trial called FOCUS4 which aimed to investigate the best ways to help people with inoperable bowel cancer who have already received some chemotherapy. This trial had significant input from researchers in the University of Glasgow and from the Beatson West of Scotland Cancer Centre.
The researchers caution that these are early results and that larger trials are needed to establish whether the drug improves survival compared to standard treatment. The trial tested adavosertib among patients who were on a treatment break following chemotherapy but the drug could potentially benefit patients with other types of bowel cancer or alongside standard treatments in other lines of therapy.
The subset of patients who took part in the trial had tumors with two common mutations, RAS and TP53, that the researchers hypothesized would make the tumors more sensitive to the effects of the drug. About a third of all colorectal cancer patients have tumors with these two mutations.
Bowel cancer is the fourth most common cancer in the UK and the second biggest cancer killer. Over 42,000 people are diagnosed with bowel cancer every year in the UK.
Professor Richard Wilson, FOCUS4 co-chief investigator from the University of Glasgow, said: “The FOCUS4 trial demonstrates how collaborative research and team science can deliver for our current and future patients with cancer. We enrolled 1,434 patients from 88 hospitals across all 4 UK devolved nations, answering important questions about how best to treat colorectal cancer in the future.
“Our local lead Dr. Janet Graham, the CRUK Clinical Trials Unit Glasgow and our clinical cancer research staff across the West of Scotland made a very significant contribution to the success of FOCUS4.”
Lead author Dr. Jenny Seligmann, of the University of Leeds, said: “These results show promising signs that adavosertib may be effective in delaying re-growth of bowel cancer in some patients and is well tolerated. The findings are particularly encouraging as the subset of patients involved represent a third of all bowel cancer patients and, while other patients have treatments developed specifically for their tumor types, this group currently has very limited treatment options.”
More than 1,400 people with bowel cancer took part in the FOCUS4 trial program. Blood samples and tumors were analyzed and some of those enrolled took part in additional randomized controlled trials that tested new drugs in people whose cancer had particular chemical changes that suggested those drugs might be effective.
Co-author Professor Louise Brown (MRC Clinical Trials Unit at UCL), statistical lead for the FOCUS4 trial, said: “Our UK-wide trial is the first in the world to investigate potential treatments for bowel cancer by stratifying patient groups according to the chemical make-up of their tumors. This allowed us to test a number of new approaches at the same time which is a more efficient way of testing treatments. The results for the adavosertib arm of the trial are potentially important and represent a glimmer of hope for patients in this group.”
Adavosertib kills cancer cells by inhibiting WEE1, a protein that helps to regulate the process of cell division in the tumor by ensuring that any DNA damage is repaired before cells divide.
Researchers believed that tumors with the mutations RAS and TP53 would be particularly sensitive to this form of attack, as these mutations have already placed the process of cell replication under stress.
FOCUS4 co-chief investigator, Professor Tim Maughan, of the University of Oxford, said: “WEE1 inhibitors target the DNA repair process in tumor cells. A similar strategy is used to treat some ovarian and breast cancers with drugs called PARP inhibitors. However, this is the first time this strategy has been successfully used to treat bowel cancer.”
Side effects of the drug included fatigue, diarrhea, neutropenia (involving low levels of white blood cells called neutrophils), and nausea, but none of these occurred in more than 11% of patients.
A second new study from a separate part of the FOCUS4 trial called FOCUS4-N, also published this week in the Journal of Clinical Oncology, looked at outcomes among patients who had a complete break from treatment following chemotherapy, comparing them to outcomes among those who continued chemotherapy using a simpler tablet called capecitabine.
The researchers found that, among those who had a complete break, the cancer started to grow somewhat sooner than in those on continued maintenance therapy, but that maintenance therapy did not lead to an increase in how long people lived.
Lead author Professor Richard Adams, of Cardiff University, said: “The findings will help to inform discussions between patients and clinicians about treatment options at the end of four months of therapy—that is, whether to stay on oral chemotherapy long-term or have a complete break in treatment—giving patients better control of their cancer management.”
Michelle Mitchell, Cancer Research UK’s chief executive, said: “While this trial was set up to explore a new drug that could help delay bowel cancer returning in patients who are on a chemo break, it’s opened up the possibility that adavosertib could also be used in patients with bowel cancer who have limited treatment options. New treatments for this group of people have the potential to be life changing and we’re looking forward to seeing the next stage of this research.”
Source: Read Full Article
