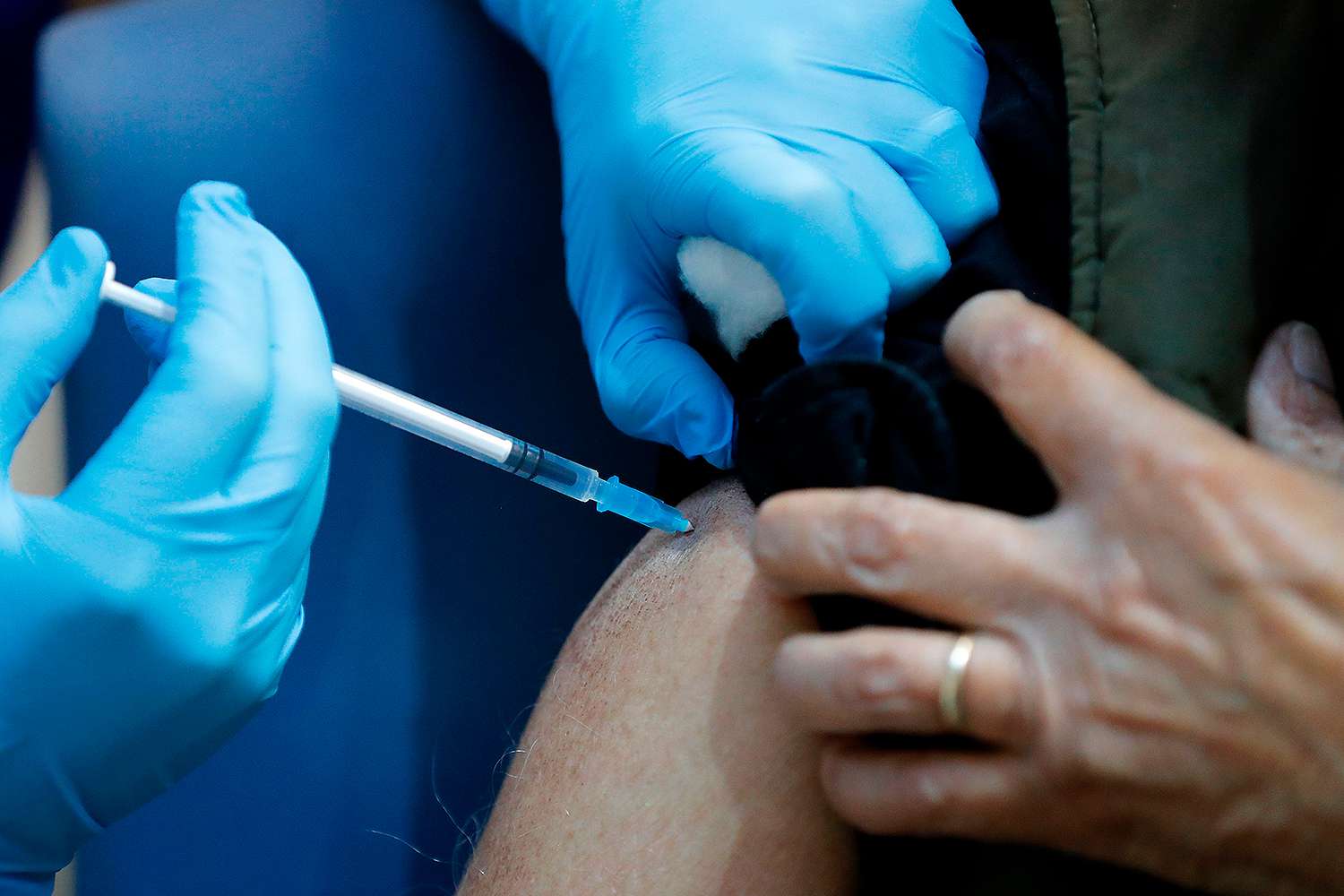
A new survey has found that over a quarter of Americans are "vaccine hesitant" about taking a shot to help prevent contracting the coronavirus.
Published Tuesday by the Kaiser Family Foundation (KFF) COVID-19 Vaccine Monitor, the findings reflect that while 71 percent of Americans would definitely or probably get a vaccine if offered — up from 63 percent in a September survey — "about a quarter (27 percent) of the public remains vaccine hesitant, saying they probably or definitely would not get a COVID-19 vaccine even if it were available for free and deemed safe by scientists."
The group went on to share that uncertainty over the vaccine was highest among Republicans (42 percent), people aged 30 to 49 (36 percent), people who live in rural areas (35 percent) and Black adults (35 percent).
Furthermore, 33 percent of American survey participants "who say they have been deemed essential workers" said they definitely or probably wouldn't opt to take the vaccine, as well as 29 percent of workers in the health-care-delivery industry.
Never miss a story — sign up for PEOPLE's free daily newsletter to stay up-to-date on the best of what PEOPLE has to offer, from juicy celebrity news to compelling human interest stories.
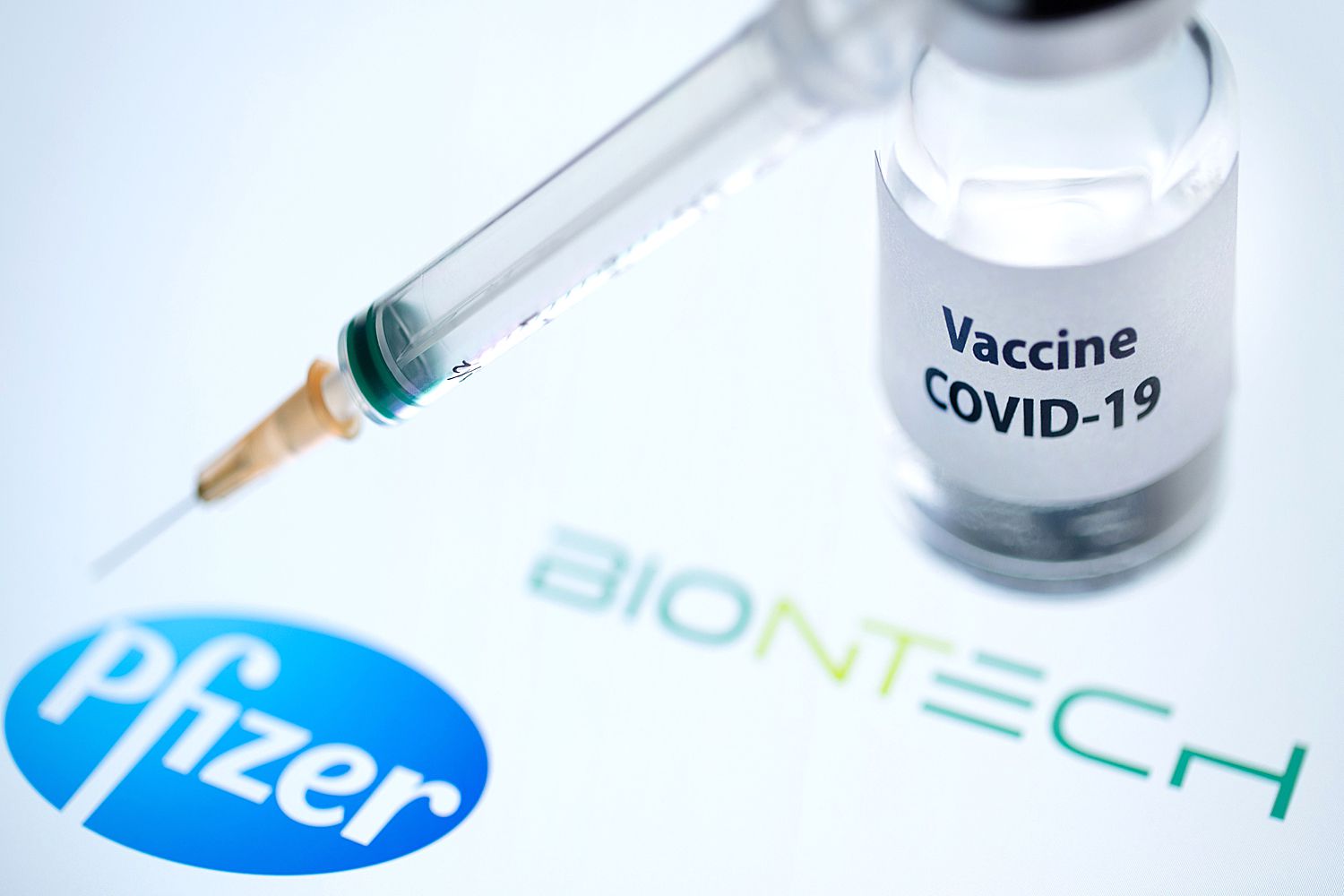
COVID Vaccinations Have Begun — Here's What to Know
"Among those who are hesitant to get a COVID-19 vaccine," the KFF states, "the main reasons are worries about possible side effects (59 percent cite this as a major reason), lack of trust in the government to ensure the vaccines' safety and effectiveness (55 percent), concerns that the vaccine is too new (53 percent) and concerns over the role of politics in the development process (51 percent)."
The group adds, "About half of Black adults who say they probably or definitely won't get vaccinated cite as major reasons that they don't trust vaccines in general (47 percent) or that they are worried they may get COVID-19 from the vaccine (50 percent), suggesting that messages combatting particular types of misinformation may be especially important for increasing vaccine confidence among this group."
On Monday, the first Americans were inoculated with Pfizer's COVID-19 vaccine — a landmark moment as the country struggles to contain the ongoing pandemic — after the two-dose vaccine was approved for emergency use by the U.S. Food and Drug Administration this past Friday.
The first vaccine went to one of the people with the highest need: an ICU nurse at Long Island Jewish Medical Center in New York City, one of the hospitals hardest hit by COVID-19 at the start of the pandemic. Over the rest of the day, week and month, millions more will receive Pfizer's vaccine.
Friday's approval meant that the Pfizer-BioNTech COVID-19 Vaccine could begin to be distributed to people 16 years and older, marking "a significant milestone in battling this devastating pandemic that has affected so many families in the United States and around the world," FDA Commissioner Stephen M. Hahn, M.D., said in a press release.
The FDA also said the "potential benefits outweigh the known and potential risks" and assured the public and medical community that "a thorough evaluation of the available safety, effectiveness and manufacturing quality information" was conducted. Notable politicians — including former Presidents Barack Obama, George W. Bush and Bill Clinton — have even volunteered to take the vaccine publicly to prove its safety to the U.S. public.
Gen. Gustave Perna said during a news conference on Saturday held by the FDA that the vaccine would arrive at 145 sites across the country on Monday. Another 425 sites were expected to receive the vaccine on Tuesday, and the final 66 sites will receive it on Wednesday, completing the initial delivery of Pfizer vaccines.
In the U.S. alone, the contagious respiratory virus has infected more than 16.6 million people, and at least 302,314 have died as a result of COVID-19 as of Tuesday afternoon, according to data from The New York Times.
Source: Read Full Article
