Alzheimer’s disease (AD) is a progressive neurological disease that causes impairments in memory and cognition. AD is believed to result from the deposition of protein “plaques” consisting of amyloid beta (Aβ) proteins sequentially generated via the enzyme γ-secretase. Although most cases of AD are sporadic or late-onset, early-onset or familial AD (FAD) caused by mutations in genes encoding γ-secretase is also noted. Differences in the sequential processing of Aβ proteins between sporadic AD and FAD have so far remained unclear.
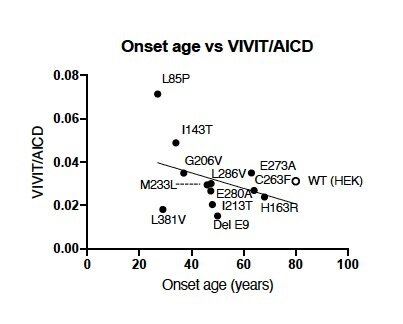
In a recent study published in Translational Psychiatry, researchers from Japan sought deeper insights into the molecular mechanisms of Aβ generation and γ-secretase activity in FAD. They systematically examined the levels of different Aβ proteins in patients with FAD caused by mutations in presenilin 1 (PS1), a gene encoding part of the γ-secretase complex. Dr. Nobuto Kakuda, who led this study, says, “The onset of FAD occurs between the ages of 40 and 60 years due to different Aβ proteins. Although certain Aβ proteins are predominantly generated in FAD, the mechanisms underlying the alterations were unclear. Our study aimed to bridge these gaps.”
Given that Aβ proteins are sequentially generated in patients with AD, the researchers first examined the concentration of Aβ38, Aβ40, Aβ42, and Aβ42 in the cerebrospinal fluid (CSF) of patients with FAD and PS1 mutations. “In the brain, there are two pathways of Aβ production. First, the amyloid precursor protein is cleaved to generate Aβ49 and Aβ48. Aβ49 is further cleaved to Aβ46 and Aβ43 and then to Aβ40. In contrast, Aβ48 is cleaved to Aβ45 and Aβ42 and then to Aβ38. So, the levels of these proteins can help us understand the status of Aβ and AD progression,” explains Dr. Kakuda. The researchers found that in some patients with FAD, although low levels of Aβ38, Aβ40, and Aβ42 were detected in the CSF, the levels of Aβ43 appeared unchanged, indicating stable Aβ43 generation.
The researchers went on to validate these findings in cultured cells expressing a mutant form of PS1. Using in vitro γ-secretase assays, they confirmed that although Aβ38, Aβ40, and Aβ42 generation is lower in cells with PS1 mutations than in wild-type cells, Aβ43 generation is unaffected. Moreover, ratio between the level of Aβ43 and the level of the amyloid precursor protein intracellular domain (AICD) was elevated in these cells, further indicating increased Aβ43 generation. Together, these findings provided strong evidence supporting their initial hypothesis—the mechanism of Aβ processing appeared to be different in FAD compared to sporadic AD.
Next, the team used liquid chromatography–mass spectrometry to understand these altered mechanisms in more detail. Their findings revealed that in PS1 mutants, Aβ43 was not generated only from Aβ46, as in sporadic AD, but also from Aβ48. “We measured the release of various peptides generated during Aβ generation using mass spectrometry. In this way, we could find the source of the additional Aβ43 generated in cases of FAD,” says Dr. Kakuda.
Source: Read Full Article